Model verification
Simulation of dynamic responses at metabolic level induced by incremental test and high-intensity intermittent exercises
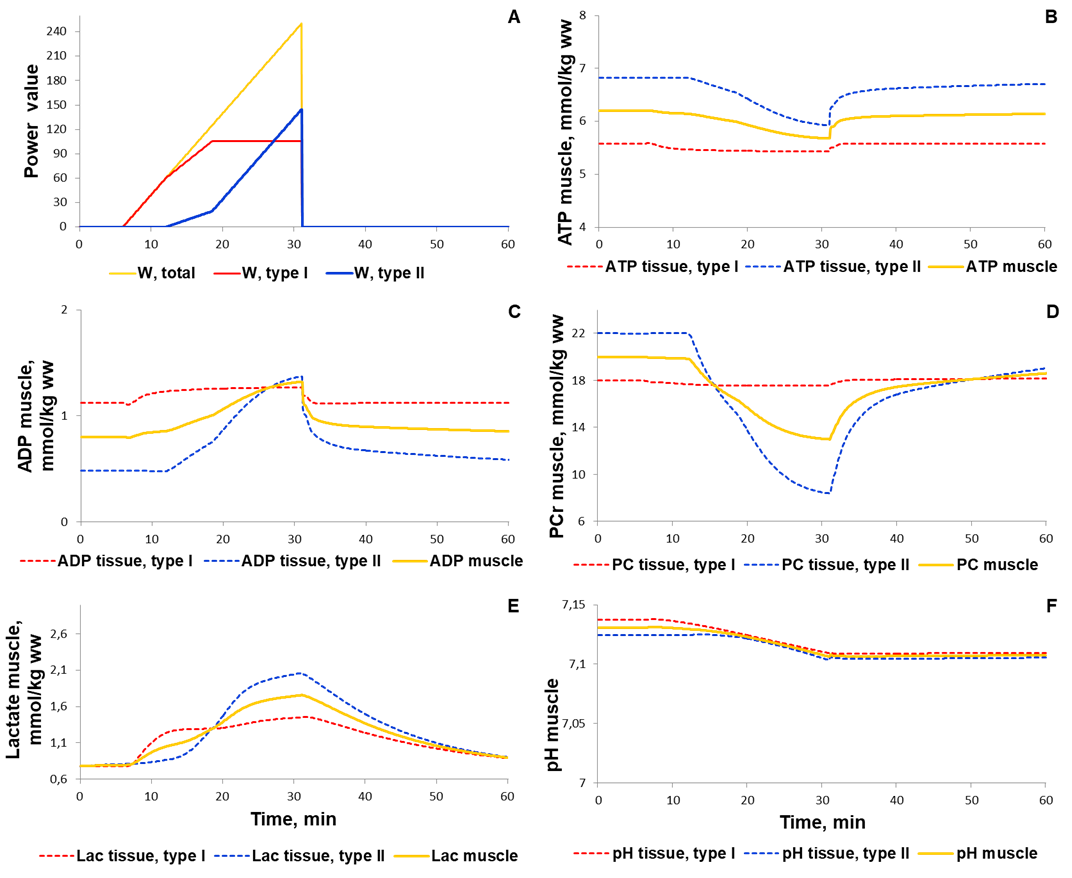
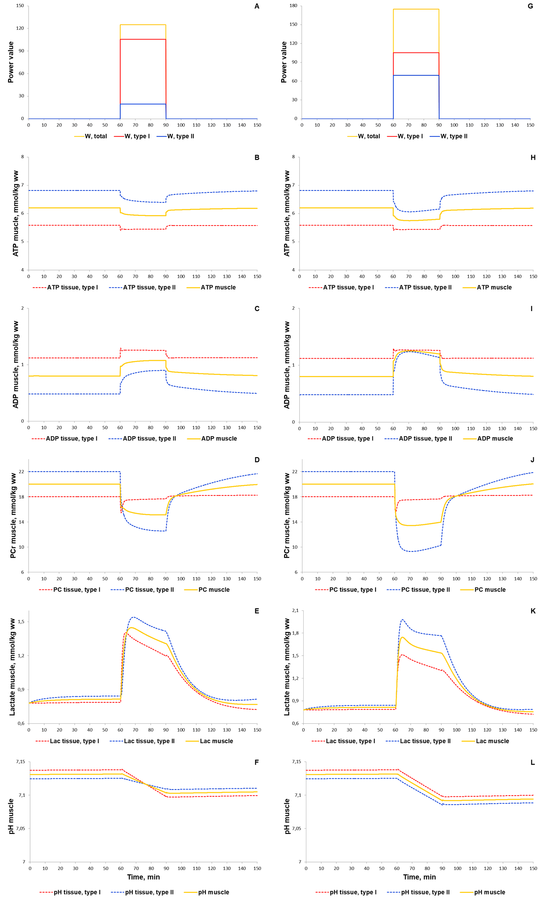
To validate the model we investigated a dynamic behaviour of the system in response to diverse single-bout aerobic exercises and compared them with published experimental data. Initially, we quantitatively estimated biochemical responses of essential metabolic variables (ATP, ADP, PCr, lactate concentrations and pH in muscle fibers type I and II) in the incremental ramp test which is a commonly used approach to evaluate aerobic capacity. In our simulation, muscle fibres type I are recruited after the beginning of exercise, while fibre type II - only at power over 24% of VO2max (6 min after exercise onset, Fig. 1A). Increasing the power during the test effects on various physiological variables like the volume of muscle containing fibre type I and/or II, blood flow as well as of transport and metabolic fluxes in both fibre types (Fig. 1). The model simulations reasonably well correspond to experimental measurements (Roussel et al., 2003; Greiner et al., 2007; Cannon et al., 2013) [1][2] [3] obtained in studies with the exercise mode (Fig. 2). However, it is worth to note that the current version of the model does not take into account an effect of the muscle exhaustion during the incremental ramp test which is physiologically observed and provides a direction for the model improvements.
Figure 1. Simulation results for incremental ramp exercise till exhaustion. (A) Exercise power and fibers recruitment pattern: total power, W (orange), power generated by type I (red) and II (blue) fibers; (B) ATP concentrations in type I (red, dotted) and II (blue, dotted) fibers and in the muscle tissue, mmol/kg ww (orange, solid); (C) ADP concentrations in type I (red, dotted) and II (blue, dotted) fibers and in the muscle tissue, mmol/kg ww (orange, solid); (D) PCr concentrations in type I (red, dotted) and II (blue, dotted) fibers and in the muscle tissue, mmol/kg ww (orange, solid); (E) Lactate concentrations in type I (red, dotted) and II (blue, dotted) fibers and in the muscle tissue, mmol/kg ww (orange, solid); (F) pH changes in type I (red, dotted) and II (blue, dotted) fibers and in the muscle tissue, mmol/kg ww (orange, solid).
|
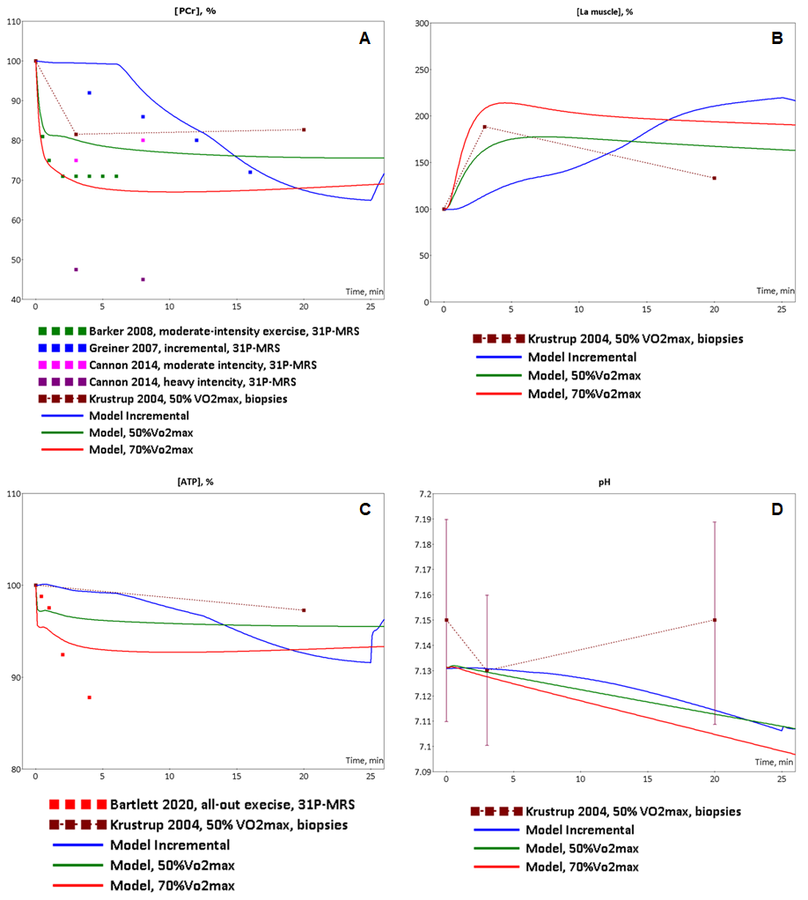
Figure 2. Model validation. The model simulations were compared to experimental data collected from incremental ramp and low intensity, moderate intensity exercises. (A) Model-predicted dynamic changes of PCr concentration in muscle tissue cells during incremental (blue line), low intensity(green line) and moderate intensity (red line) exercises (axis X - time of the exercise in minutes) and experimental measurements of PCr concentration changes during incremental (blue dots, Greiner et al., 2007)[2], low-intensity (dark red dots (Krustrup et al., 2004)[4], green dots (Barker et al., 2008)[5], pink dots (Cannon et al., 2014)[6]) and high-intensity (dark pink dots (Cannon et al., 2014)[6] exercises; (B) Model-predicted dynamic changes of lactate concentration in muscle tissue cells during incremental (blue line), low intensity(green line) and moderate intensity (red line) exercises (axis X - time of the exercise in minutes) and experimental measurements of lactate concentration changes during low-intensity (dark red dots (Krustrup et al., 2004)[4]) exercise; (C) Model-predicted dynamic changes of ATP concentration in muscle tissue cells during incremental (blue line), low intensity(green line) and moderate intensity (red line) exercises (axis X - time of the exercise in minutes) and experimental measurements of ATP concentration changes during low-intensity (dark red dots (Krustrup et al., 2004)[4]) and all-out intensity (red dots (Bartlett et al., 2020)[7]) exercises; (D) Model-predicted dynamic changes of pH in muscle tissue cells during incremental (blue line), low intensity(green line) and moderate intensity (red line) exercises (axis X - time of the exercise in minutes) and experimental measurements of ATP concentration changes during low-intensity (dark red dots (means ± SD) (Krustrup et al., 2004)[4]) exercise.
|
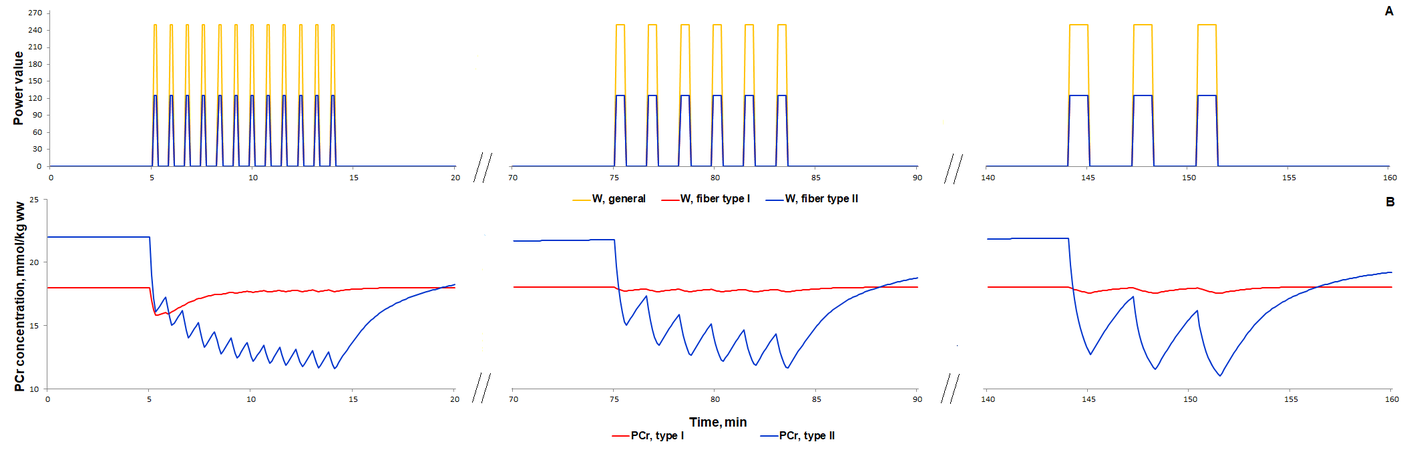
For additional verification of the model, we simulated responses to an alternative exercise mode that is a high-intensity intermittent exercise (Fig. 3, 4). As a result, the model qualitatively reproduces dynamic changes in PCr concentration during high-intensity intermittent exercise with different work:recovery durations.
Figure 3. Simulation results for high-intensity intermittent exercise with different work:recovery durations (initial - 16:32 s; intermediate - 32:64 s; final - 64:128 s) (Davies et al., 2017)[8]. (A) Exercise power and fibers recruitment pattern: total power (Wmax=250), W (orange), power generated by type I (red, Wmax=125) and II (blue, Wmax=125) fibers; (B) PCr concentration (mmol/kg ww) in Type I (red) and Type II fibers (blue).
|
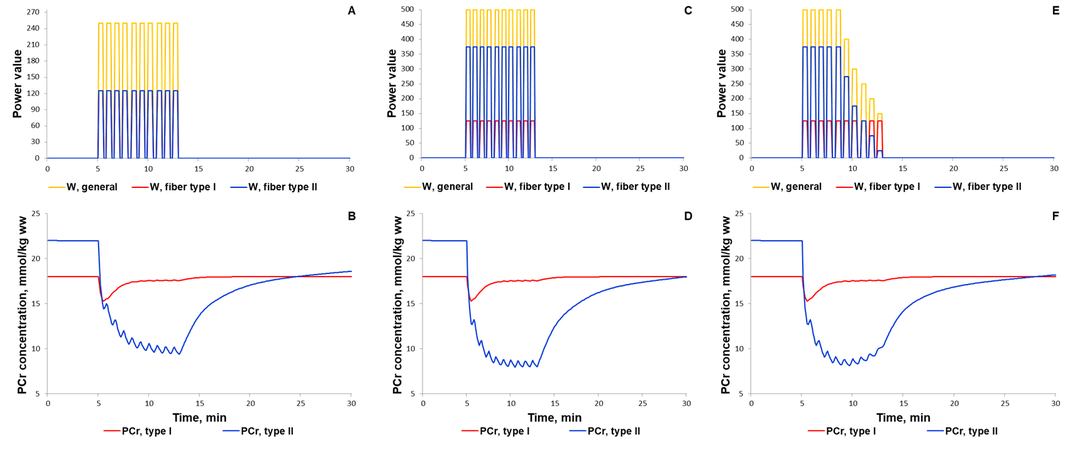
The first scenario to reproduce experimental PCr measurements in Kappenstein with coathours study (Kappenstein et al., 2013)[9] used the external power which is equal to 250, while the parameter value was doubled in the second simulated scenario. The maximal value of the parameter was unchangeable on each bout of 30-s exercise separated by 20-s recovery. Whereas the external power was gradually decreased after the fifth bout (Fig. 4E) due to physical exhaustion of the volunteered subject. It has been predicted that gradual exhaustion or reduction in the external power after a certain exercise bout much better reproduces experimental measurements on phosphocreatine dynamics in skeletal muscle than simulations with constant maximal power value on each bout (Kappenstein et al., 2013)[9].
Figure 4. Simulation results for high-intensity intermittent exercise (Kappenstein et al., 2013)[9]. (A, C, E) Exercise power and fibers recruitment pattern: total power (A: Wmax=250; C: Wmax=500; E: Wmax=500), W (orange), power generated by type I (red, A-C: Wmax=125) and II (blue, A: Wmax=125; C: Wmax=375; E: Wmax=375 and successive decline) fibers; (B) PCr concentration (mmol/kg ww) in Type I (red) and Type II fibers (blue) with total power at Wmax=250; (D) PCr concentration (mmol/kg ww) in Type I (red) and Type II fibers (blue) with total power at Wmax=500; (F) PCr concentration (mmol/kg ww) in Type I (red) and Type II fibers (blue) with total power at Wmax=500 and successive decline of the power on each bout of 30-s exercise.
|
Simulation of dynamic responses at metabolic and gene regulatory levels induced by low- and moderate intensity exercises
At the next step of the model validation, we predicted dynamic changes of the same biochemical variables, an activation of both signaling molecules (AMPK and Ca2+-dependent proteins) and transcription factor (CREB1), as well as expression of genes (NR4A3, NR4A2, PPARGC1A) in response to low (50% VO2max) and moderate intensity (70% VO2max) continuous exercises. During this type of the exercise mode metabolic fluxes varied by means of recruitment of both muscle fibers type I, and type II. Comparison of the model simulations with experimental data (Krustrup et al., 2004; Barker et al., 2008; Cannon et al., 2014; Fiedler et al., 2016; Bartlett et al., 2020)[4][5][6][10][7] has shown that the model reasonably well reproduces dynamic behaviour of the muscle metabolism during the exercise (Fig. 2 and 5).
Figure 5. Simulation results for low (50% VO2max, A-F) and moderate intensity (70% VO2max, G-L) continuous exercises. (A,G) Exercise power and fibers recruitment pattern: total power, W (orange), power generated by type I (red) and II (blue) fibers; (B,H) ATP concentrations in type I (red, dotted) and II (blue, dotted) fibers and in the muscle tissue, mmol/kg ww (orange, solid); (C,I) ADP concentrations in type I (red, dotted) and II (blue, dotted) fibers and in the muscle tissue, mmol/kg ww (orange, solid); (D,J) PCr concentrations in type I (red, dotted) and II (blue, dotted) fibers and in the muscle tissue, mmol/kg ww (orange, solid); (E,K) Lactate concentrations in type I (red, dotted) and II (blue, dotted) fibers and in the muscle tissue, mmol/kg ww (orange, solid); (F,L) pH changes in type I (red, dotted) and II (blue, dotted) fibers and in the muscle tissue, mmol/kg ww (orange, solid).
|
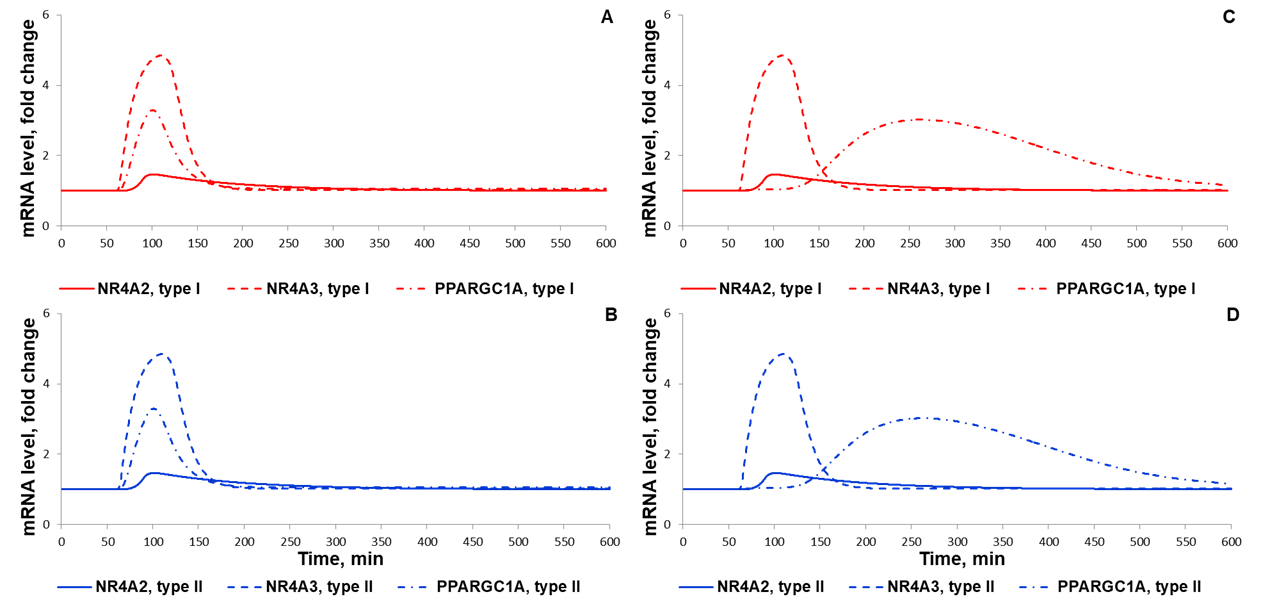
Development of the integrated model incorporating signaling and gene expression modules enables to simulate expression dynamics of early and delayed response genes on mRNA levels (Fig. 6). Numerical analysis of the model enabled to reveal crucial steps in this signal transduction pathway for the adaptation and demonstrated the necessity of consideration of additional transcription factors modulating transcription of genes with delayed response in order to adequately reproduce gene expression data that were taken in human vastus lateralis muscle during and after acute cycling exercise (Fig. 6C-D). Bioinformatics analysis of the original transcriptomics data, in turn, proposed that CREB-like proteins from FOS and JUN families forming heterodimer complexes with transcription factor CREB1 are indeed these intermediate regulators of genes with delayed response (Akberdin et al., 2020).
Figure 6. Simulation results for moderate intensity (70% VO2max) continuous exercises without (A-B) and with (C-D) intermediate X factor regulating expression of PPARGC1A gene. Expression of (A,C) NR4A3 (solid), NR4A2 (dashed), PPARGC1A (dot-dashed) in Type I fibers, (B,D) the same indexes in type II fibers (in Fold changes).
|
Discussion
The Integrated modular model comprises three hierarchical levels (metabolic, signaling pathways and regulation of gene expression)
Model constraints and further ways for the development
References
- Kolpakov F, Akberdin I, Kashapov T, Kiselev L, Kolmykov S, Kondrakhin Y, Kutumova E, Mandrik N, Pintus S, Ryabova A, Sharipov R, Yevshin I, and Kel A. BioUML: an integrated environment for systems biology and collaborative analysis of biomedical data. Nucleic Acids Res. 2019 Jul 2;47(W1):W225-W233. DOI:10.1093/nar/gkz440 | PubMed ID:31131402 | HubMed [1]