Difference between revisions of "CaMKKβ"
(→Structure) |
(→Summary remarks) |
||
| (3 intermediate revisions by the same user not shown) | |||
| Line 2: | Line 2: | ||
https://www.genecards.org/cgi-bin/carddisp.pl?gene=CAMKK2 | https://www.genecards.org/cgi-bin/carddisp.pl?gene=CAMKK2 | ||
| + | |||
===Summary remarks=== | ===Summary remarks=== | ||
| Line 7: | Line 8: | ||
The Ca2+/calmodulin-dependent protein kinase kinase β (CaMKKβ) likely phosphorylates and activates AMPKα1 complexes during long-term low intensity exercise/contraction (Jensen 2007 <cite>1</cite>, Treebak 2007 <cite>4</cite>), see review (Kjøbsted 2018) <cite>3</cite>. | The Ca2+/calmodulin-dependent protein kinase kinase β (CaMKKβ) likely phosphorylates and activates AMPKα1 complexes during long-term low intensity exercise/contraction (Jensen 2007 <cite>1</cite>, Treebak 2007 <cite>4</cite>), see review (Kjøbsted 2018) <cite>3</cite>. | ||
| + | |||
Nevertheless, no data exists on CaMKKβ action in human skeletal muscle up to date. The data links from (Kjøbsted 2018) do not supports such speculation about CaMKKβ action during exercise. | Nevertheless, no data exists on CaMKKβ action in human skeletal muscle up to date. The data links from (Kjøbsted 2018) do not supports such speculation about CaMKKβ action during exercise. | ||
| Line 12: | Line 14: | ||
Recently, AMPKα was shown to be an additional substrate of CaMKK2, see review (Racioppi 2012) <cite>6</cite>. Phosphorylation and activation of AMPK occur in response to an increase in intracellular Ca2+ in LKB1 (liver kinase B1)-deficient cells, and this effect is dependent on CaMKK2 (Hawley 2005) <cite>2</cite>. Down-regulation of CaMKK2 in mammalian cells using RNA interference almost completely abolishes AMPK activation (Woods 2005) <cite>7</cite>. Finally, purified CaMKK2 will phosphorylate and activate AMPKα in vitro <cite>7</cite>, and these two kinases form a stable multiprotein complex consisting of Ca2+/CaM, CaMKK2, AMPKα, and AMPKβ (Anderson 2008) <cite>8</cite>, which is regulated by Ca2+, but not by AMP, due to the absence of the AMP-binding subunit. Green et al. 2011 <cite>9</cite> demonstrated that CaMKK2 and AMPK associate through their kinase domains and found that CaMKK2must be in an active conformation to bind AMPK, but not to associate with other substrates, such as CaMKIV. These findings suggest the hypothesis that signals modifying the activation status of CaMKK2 may act as molecular switches to couple CaMKK2 with AMPK. | Recently, AMPKα was shown to be an additional substrate of CaMKK2, see review (Racioppi 2012) <cite>6</cite>. Phosphorylation and activation of AMPK occur in response to an increase in intracellular Ca2+ in LKB1 (liver kinase B1)-deficient cells, and this effect is dependent on CaMKK2 (Hawley 2005) <cite>2</cite>. Down-regulation of CaMKK2 in mammalian cells using RNA interference almost completely abolishes AMPK activation (Woods 2005) <cite>7</cite>. Finally, purified CaMKK2 will phosphorylate and activate AMPKα in vitro <cite>7</cite>, and these two kinases form a stable multiprotein complex consisting of Ca2+/CaM, CaMKK2, AMPKα, and AMPKβ (Anderson 2008) <cite>8</cite>, which is regulated by Ca2+, but not by AMP, due to the absence of the AMP-binding subunit. Green et al. 2011 <cite>9</cite> demonstrated that CaMKK2 and AMPK associate through their kinase domains and found that CaMKK2must be in an active conformation to bind AMPK, but not to associate with other substrates, such as CaMKIV. These findings suggest the hypothesis that signals modifying the activation status of CaMKK2 may act as molecular switches to couple CaMKK2 with AMPK. | ||
| + | |||
Despite this reports, (Fogarty 2010) <cite>11</cite> could not find any evidence that the α and/or β subunits of AMPK formed a stable complex with CaMKKβ. | Despite this reports, (Fogarty 2010) <cite>11</cite> could not find any evidence that the α and/or β subunits of AMPK formed a stable complex with CaMKKβ. | ||
| + | |||
(Nakanishi 2017) <cite>16</cite> show that AMPK-mediated feedback phosphorylation of CaMKKβ regulates the CaMKKβ /AMPK signaling cascade and may be physiologically important for intracellular maintenance of Ca2+ -dependent AMPK activation by CaMKKβ. | (Nakanishi 2017) <cite>16</cite> show that AMPK-mediated feedback phosphorylation of CaMKKβ regulates the CaMKKβ /AMPK signaling cascade and may be physiologically important for intracellular maintenance of Ca2+ -dependent AMPK activation by CaMKKβ. | ||
| − | + | ||
| + | The most of CaMKKβ studies used various cell lines or in vitro models (<cite>2</cite>, <cite>5</cite>, <cite>7</cite>, <cite>8</cite>, <cite>9</cite>, <cite>10</cite>, <cite>11</cite>, <cite>13</cite>, <cite>14</cite>, <cite>16</cite>, <cite>17</cite>. Therefore these data must be taken in consideration with caution, when used directly for modeling of skeletal muscles. | ||
The study on contracting perfused rat skeletal muscle show the importance of Ca2+-dependent signaling via CaMKK activation in the regulation of substrate uptake and FA oxidation and agree with the notion that CaMKK is an upstream kinase of AMPK in the regulation of substrate metabolism in skeletal muscle during 20 minutes of moderate-intensity muscle contraction (Abbott 2009) <cite>12</cite>. | The study on contracting perfused rat skeletal muscle show the importance of Ca2+-dependent signaling via CaMKK activation in the regulation of substrate uptake and FA oxidation and agree with the notion that CaMKK is an upstream kinase of AMPK in the regulation of substrate metabolism in skeletal muscle during 20 minutes of moderate-intensity muscle contraction (Abbott 2009) <cite>12</cite>. | ||
| + | |||
CaMKKs act in mouse skeletal muscle regulating AMPK phosphorylation and glucose uptake at the onset of mild tetanic contraction and that an intensity- and/or time-dependent switch occurs in the relative importance of AMPKKs during contraction (Jensen 2007) <cite>1</cite>. The most effect of CaMKKβ on AMPK phosphorylation takes place at the first minutes of contraction (about 2 minutes) then this effect was blunted. | CaMKKs act in mouse skeletal muscle regulating AMPK phosphorylation and glucose uptake at the onset of mild tetanic contraction and that an intensity- and/or time-dependent switch occurs in the relative importance of AMPKKs during contraction (Jensen 2007) <cite>1</cite>. The most effect of CaMKKβ on AMPK phosphorylation takes place at the first minutes of contraction (about 2 minutes) then this effect was blunted. | ||
| Line 53: | Line 59: | ||
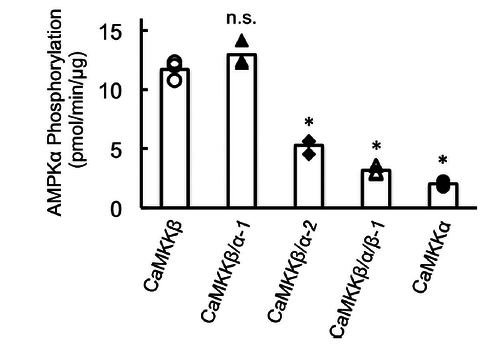
In vitro phosphorylation of AMPK by recombinant CaMKK isoforms (Fujiwara 2016) <cite>13</cite>. | In vitro phosphorylation of AMPK by recombinant CaMKK isoforms (Fujiwara 2016) <cite>13</cite>. | ||
| − | [[File: In_vitro_phosphorylation_of_AMPK_by_recombinant_CaMKK_isoforms_(Fujiwara_2016).png | | + | [[File: In_vitro_phosphorylation_of_AMPK_by_recombinant_CaMKK_isoforms_(Fujiwara_2016).png | 500px | In vitro phosphorylation of AMPK by recombinant CaMKK isoforms]] |
Substrate phosphorylation by CaMKK catalytic domain, including wild-type and chimera mutants <cite>13</cite>. | Substrate phosphorylation by CaMKK catalytic domain, including wild-type and chimera mutants <cite>13</cite>. | ||
| − | [[File: Substrate_phosphorylation_by_CaMKK_catalytic_domain,_including_wild-type_and_chimera_mutants_(Fujiwara_2016).png | | + | [[File: Substrate_phosphorylation_by_CaMKK_catalytic_domain,_including_wild-type_and_chimera_mutants_(Fujiwara_2016).png | 500px | Substrate phosphorylation by CaMKK catalytic domain, including wild-type and chimera mutants]] |
Latest revision as of 02:42, 7 October 2018
CaMKKβ. Calcium/Calmodulin Dependent Protein Kinase Kinase 2. Ver. 0.7.
https://www.genecards.org/cgi-bin/carddisp.pl?gene=CAMKK2
Summary remarks
The Ca2+/calmodulin-dependent protein kinase kinase β (CaMKKβ) likely phosphorylates and activates AMPKα1 complexes during long-term low intensity exercise/contraction (Jensen 2007 [1], Treebak 2007 [2]), see review (Kjøbsted 2018) [3].
Nevertheless, no data exists on CaMKKβ action in human skeletal muscle up to date. The data links from (Kjøbsted 2018) do not supports such speculation about CaMKKβ action during exercise.
Calcium/calmodulin-dependent protein kinase belonging to a proposed calcium-triggered signaling cascade involved in a number of cellular processes. Isoform 1, isoform 2 and isoform 3 phosphorylate CAMK1 and CAMK4. Isoform 3 phosphorylates CAMK1D. Isoform 4, isoform 5 and isoform 6 lacking part of the calmodulin-binding domain are inactive. Efficiently phosphorylates 5'-AMP-activated protein kinase (AMPK) trimer, including that consisting of PRKAA1, PRKAB1 and PRKAG1. This phosphorylation is stimulated in response to Ca2+ signals.
Recently, AMPKα was shown to be an additional substrate of CaMKK2, see review (Racioppi 2012) [4]. Phosphorylation and activation of AMPK occur in response to an increase in intracellular Ca2+ in LKB1 (liver kinase B1)-deficient cells, and this effect is dependent on CaMKK2 (Hawley 2005) [5]. Down-regulation of CaMKK2 in mammalian cells using RNA interference almost completely abolishes AMPK activation (Woods 2005) [6]. Finally, purified CaMKK2 will phosphorylate and activate AMPKα in vitro [6], and these two kinases form a stable multiprotein complex consisting of Ca2+/CaM, CaMKK2, AMPKα, and AMPKβ (Anderson 2008) [7], which is regulated by Ca2+, but not by AMP, due to the absence of the AMP-binding subunit. Green et al. 2011 [8] demonstrated that CaMKK2 and AMPK associate through their kinase domains and found that CaMKK2must be in an active conformation to bind AMPK, but not to associate with other substrates, such as CaMKIV. These findings suggest the hypothesis that signals modifying the activation status of CaMKK2 may act as molecular switches to couple CaMKK2 with AMPK.
Despite this reports, (Fogarty 2010) [9] could not find any evidence that the α and/or β subunits of AMPK formed a stable complex with CaMKKβ.
(Nakanishi 2017) [10] show that AMPK-mediated feedback phosphorylation of CaMKKβ regulates the CaMKKβ /AMPK signaling cascade and may be physiologically important for intracellular maintenance of Ca2+ -dependent AMPK activation by CaMKKβ.
The most of CaMKKβ studies used various cell lines or in vitro models ([5], [11], [6], [7], [8], [12], [9], [13], [14], [10], [15]. Therefore these data must be taken in consideration with caution, when used directly for modeling of skeletal muscles.
The study on contracting perfused rat skeletal muscle show the importance of Ca2+-dependent signaling via CaMKK activation in the regulation of substrate uptake and FA oxidation and agree with the notion that CaMKK is an upstream kinase of AMPK in the regulation of substrate metabolism in skeletal muscle during 20 minutes of moderate-intensity muscle contraction (Abbott 2009) [16].
CaMKKs act in mouse skeletal muscle regulating AMPK phosphorylation and glucose uptake at the onset of mild tetanic contraction and that an intensity- and/or time-dependent switch occurs in the relative importance of AMPKKs during contraction (Jensen 2007) [1]. The most effect of CaMKKβ on AMPK phosphorylation takes place at the first minutes of contraction (about 2 minutes) then this effect was blunted.
So suggested CaMKKβ action in human skeletal muscles described in (Kjøbsted 2018) [3] remains elusive.
Structure
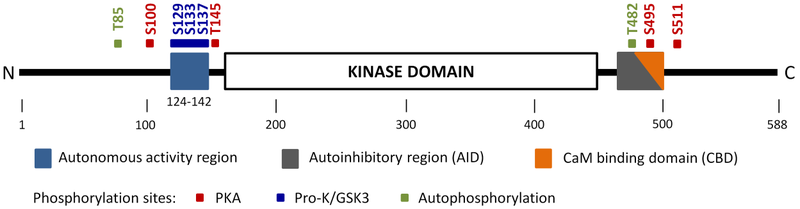
Domain organization of CaMKK2 (Kylarova 2018) [17].
The N-terminal Ca2+/CaM-autonomous activity region (residues 124-142) regulates CaMKK2 activity in the absence of Ca2+/CaM. The AID, which overlaps with the CBD, blocks substrate binding to the kinase domain and/or affects the structure of the catalytic site at low intracellular Ca2+ concentrations. Ca2+/CaM binding disrupts the interaction between the AID and the kinase domain and relieves the autoinhibition. The positions of regulatory phosphorylation and autophosphorylation sites are marked by colored squares. PKA, cAMP-dependent protein kinase; GSK3, glycogen synthase kinase-3; pro-K, proline-directed kinases.
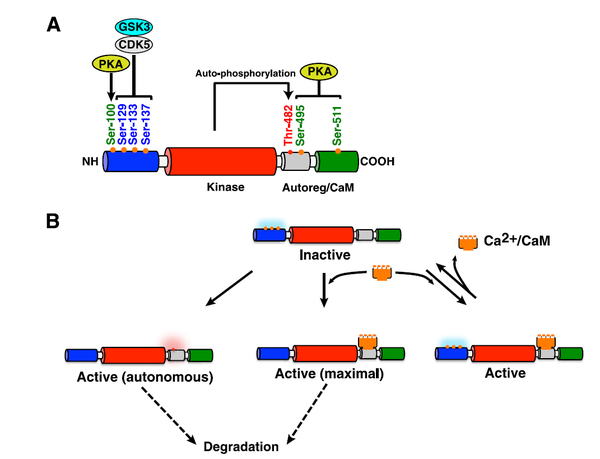
The structure-activity relationships of CaMKK2
Figure 1 [4]. Schematic representation of the structure-activity relationships of CaMKK2. A, CaMKK2 consists of unique N- and C-terminal domains (blue and green, respectively) and a central Ser/Thr-directed kinase domain (red) that is followed by a regulatory domain composed of overlapping autoinhibitory and CaM-binding regions (Autoreg/CaM, gray). A region of 23 amino acids (residues 129–151) located at the N terminus of the catalytic domain has been identified as an important regulatory element. CDK5 and GSK3 phosphorylate Ser-129, Ser-133, and Ser-137. PKA phosphorylates Ser-100, Ser-495, and Ser-511. Thr-482 has been identified as an auto/transphosphorylation site. B, although CaMKK2 has autonomous activity for some substrates, binding to Ca2+/CaM relieves autoinhibition, resulting in a fully active kinase. Mutation of Ser-129, Ser-133, and Ser-137 increases autonomous activity with little change in Ca2+/CaM-dependent activity. Of note, mutation of Ser-129, Ser-133, and Ser-137 also decreases the stability of CaMKK2. This implies that the autonomously active CaMKK2 generated by dephosphorylation of these Ser residues would display a shorter half-life and be more rapidly degraded. Mutation of PKA residues does not affect CaMKK2 autonomous activity. Phosphorylation of Ser and Thr residues is depicted as blue and red clouds, respectively.
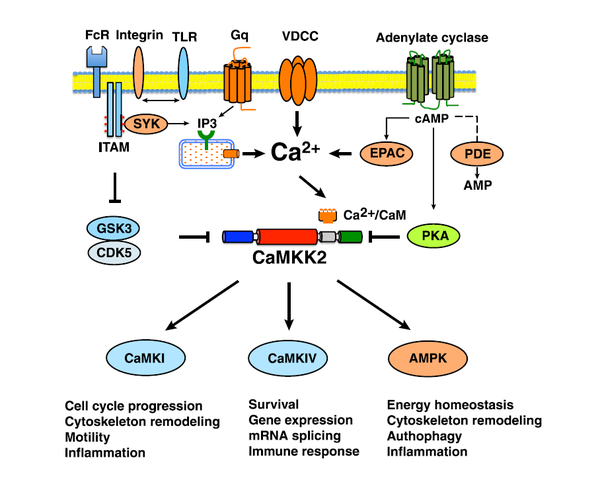
Action
CaMKK2 functions as a molecular hub to regulate critical cell functions.
Figure 2 [4]. CaMKK2 functions as a molecular hub to regulate critical cell functions. CaMKK2 can be activated by signaling through Gq-coupled receptors, IP3-mediated release of Ca2+ via activation of the IP3 receptor, or Ca2+ entry into cells via plasma membrane ion channels (i.e. voltage-dependent calcium channel (VDCC)). Calcium signals from integrin and other immunoreceptor tyrosine-based activation motif (ITAM)-coupled immune receptors can activate IP3-mediated release of Ca2+, activate CaMKK2, and cross-regulate heterologous receptors, such as the Toll-like receptors (TLR). Signaling pathways modulating GSK3/CDK5 activities can modulate the stability and autonomous activity of CaMKK2. Signals regulating the intracellular level of cAMP can activate EPAC/PKA-dependent pathways and, in turn, regulate CaMKK2 activity. CaMKI, CaMKIV, and AMPK couple CaMKK2 to multiple downstream cascades, which conspire to regulate critical cell functions. FcR, Fc receptor; PDE, phosphodiesterase.
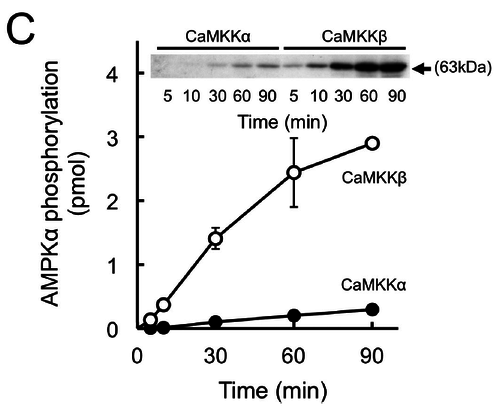
Kinetics
In vitro phosphorylation of AMPK by recombinant CaMKK isoforms (Fujiwara 2016) [13].
Substrate phosphorylation by CaMKK catalytic domain, including wild-type and chimera mutants [13].
References
- Jensen TE, Rose AJ, Jørgensen SB, Brandt N, Schjerling P, Wojtaszewski JF, and Richter EA. Possible CaMKK-dependent regulation of AMPK phosphorylation and glucose uptake at the onset of mild tetanic skeletal muscle contraction. Am J Physiol Endocrinol Metab. 2007 May;292(5):E1308-17. DOI:10.1152/ajpendo.00456.2006 |
- Treebak JT, Birk JB, Rose AJ, Kiens B, Richter EA, and Wojtaszewski JF. AS160 phosphorylation is associated with activation of alpha2beta2gamma1- but not alpha2beta2gamma3-AMPK trimeric complex in skeletal muscle during exercise in humans. Am J Physiol Endocrinol Metab. 2007 Mar;292(3):E715-22. DOI:10.1152/ajpendo.00380.2006 |
- Kjøbsted R, Hingst JR, Fentz J, Foretz M, Sanz MN, Pehmøller C, Shum M, Marette A, Mounier R, Treebak JT, Wojtaszewski JFP, Viollet B, and Lantier L. AMPK in skeletal muscle function and metabolism. FASEB J. 2018 Apr;32(4):1741-1777. DOI:10.1096/fj.201700442R |
- Racioppi L and Means AR. Calcium/calmodulin-dependent protein kinase kinase 2: roles in signaling and pathophysiology. J Biol Chem. 2012 Sep 14;287(38):31658-65. DOI:10.1074/jbc.R112.356485 |
- Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, and Hardie DG. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005 Jul;2(1):9-19. DOI:10.1016/j.cmet.2005.05.009 |
- Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, Carlson M, and Carling D. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005 Jul;2(1):21-33. DOI:10.1016/j.cmet.2005.06.005 |
- Anderson KA, Ribar TJ, Lin F, Noeldner PK, Green MF, Muehlbauer MJ, Witters LA, Kemp BE, and Means AR. Hypothalamic CaMKK2 contributes to the regulation of energy balance. Cell Metab. 2008 May;7(5):377-88. DOI:10.1016/j.cmet.2008.02.011 |
- Green MF, Anderson KA, and Means AR. Characterization of the CaMKKβ-AMPK signaling complex. Cell Signal. 2011 Dec;23(12):2005-12. DOI:10.1016/j.cellsig.2011.07.014 |
- Fogarty S, Hawley SA, Green KA, Saner N, Mustard KJ, and Hardie DG. Calmodulin-dependent protein kinase kinase-beta activates AMPK without forming a stable complex: synergistic effects of Ca2+ and AMP. Biochem J. 2010 Jan 27;426(1):109-18. DOI:10.1042/BJ20091372 |
- Nakanishi A, Hatano N, Fujiwara Y, Sha'ri A, Takabatake S, Akano H, Kanayama N, Magari M, Nozaki N, and Tokumitsu H. AMP-activated protein kinase-mediated feedback phosphorylation controls the Ca(2+)/calmodulin (CaM) dependence of Ca(2+)/CaM-dependent protein kinase kinase β. J Biol Chem. 2017 Dec 1;292(48):19804-19813. DOI:10.1074/jbc.M117.805085 |
- Hawley SA, Selbert MA, Goldstein EG, Edelman AM, Carling D, and Hardie DG. 5'-AMP activates the AMP-activated protein kinase cascade, and Ca2+/calmodulin activates the calmodulin-dependent protein kinase I cascade, via three independent mechanisms. J Biol Chem. 1995 Nov 10;270(45):27186-91. DOI:10.1074/jbc.270.45.27186 |
- Yurimoto S, Fujimoto T, Magari M, Kanayama N, Kobayashi R, and Tokumitsu H. In vitro substrate phosphorylation by Ca²⁺/calmodulin-dependent protein kinase kinase using guanosine-5'-triphosphate as a phosphate donor. BMC Biochem. 2012 Dec 5;13:27. DOI:10.1186/1471-2091-13-27 |
- Fujiwara Y, Kawaguchi Y, Fujimoto T, Kanayama N, Magari M, and Tokumitsu H. Differential AMP-activated Protein Kinase (AMPK) Recognition Mechanism of Ca2+/Calmodulin-dependent Protein Kinase Kinase Isoforms. J Biol Chem. 2016 Jun 24;291(26):13802-8. DOI:10.1074/jbc.M116.727867 |
- Hurley RL, Anderson KA, Franzone JM, Kemp BE, Means AR, and Witters LA. The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J Biol Chem. 2005 Aug 12;280(32):29060-6. DOI:10.1074/jbc.M503824200 |
- Shen QW, Zhu MJ, Tong J, Ren J, and Du M. Ca2+/calmodulin-dependent protein kinase kinase is involved in AMP-activated protein kinase activation by alpha-lipoic acid in C2C12 myotubes. Am J Physiol Cell Physiol. 2007 Oct;293(4):C1395-403. DOI:10.1152/ajpcell.00115.2007 |
- Abbott MJ, Edelman AM, and Turcotte LP. CaMKK is an upstream signal of AMP-activated protein kinase in regulation of substrate metabolism in contracting skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2009 Dec;297(6):R1724-32. DOI:10.1152/ajpregu.00179.2009 |
- Kylarova S, Psenakova K, Herman P, Obsilova V, and Obsil T. CaMKK2 kinase domain interacts with the autoinhibitory region through the N-terminal lobe including the RP insert. Biochim Biophys Acta Gen Subj. 2018 Oct;1862(10):2304-2313. DOI:10.1016/j.bbagen.2018.07.025 |