Difference between revisions of "Integrated model validation"
(→Discussion) |
(→References) |
||
| Line 83: | Line 83: | ||
===References=== | ===References=== | ||
<biblio> | <biblio> | ||
| + | #1 pmid=12433845 <!-- Roussel et al 2003 --> | ||
| + | #1 pmid=31131402 <!-- Kolpakov et al 2019 --> | ||
#1 pmid=31131402 <!-- Kolpakov et al 2019 --> | #1 pmid=31131402 <!-- Kolpakov et al 2019 --> | ||
Revision as of 17:07, 25 March 2021
Contents
Model verification
Simulation of dynamic responses at metabolic level induced by incremental test and high-intensity intermittent exercises
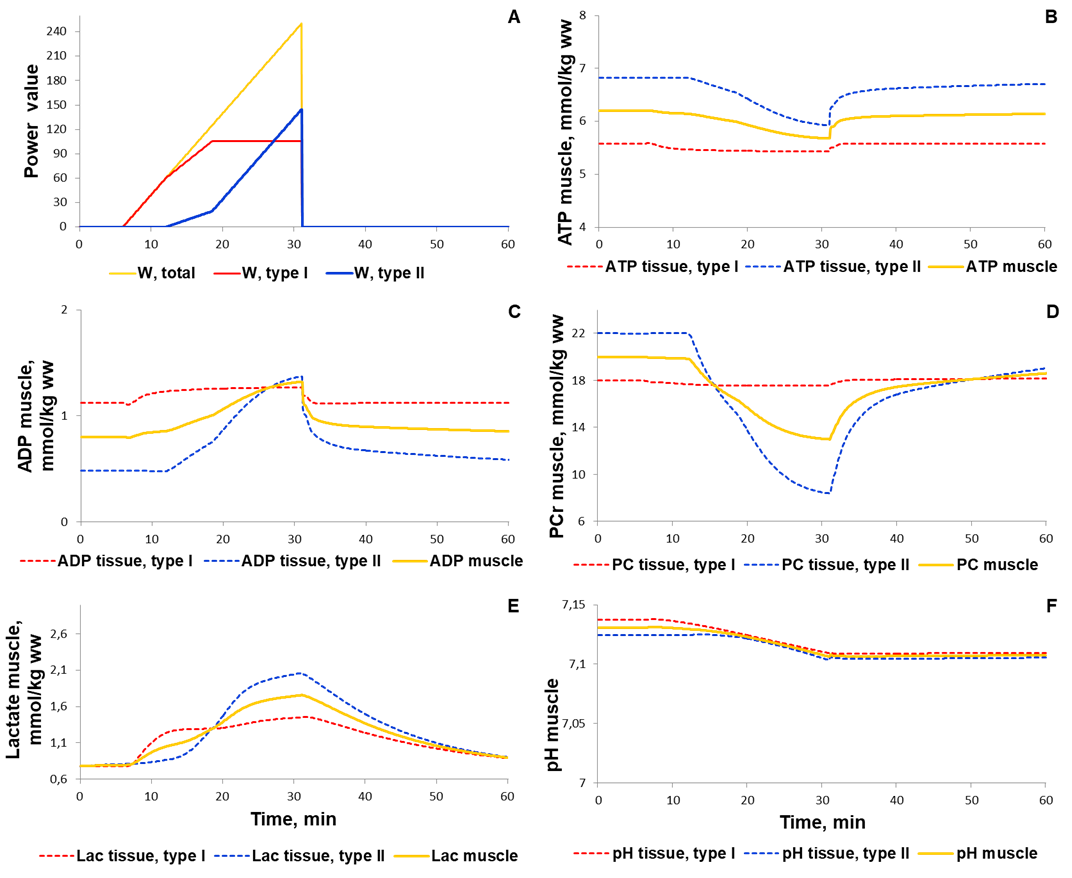
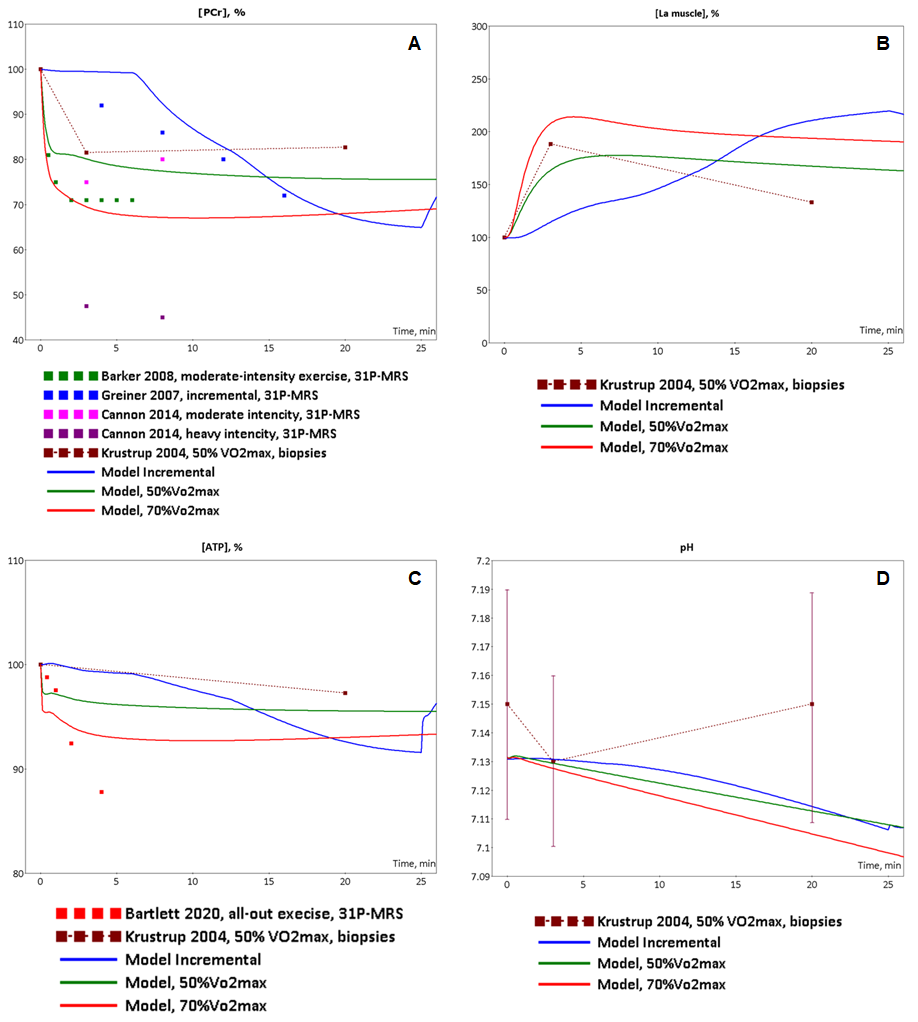
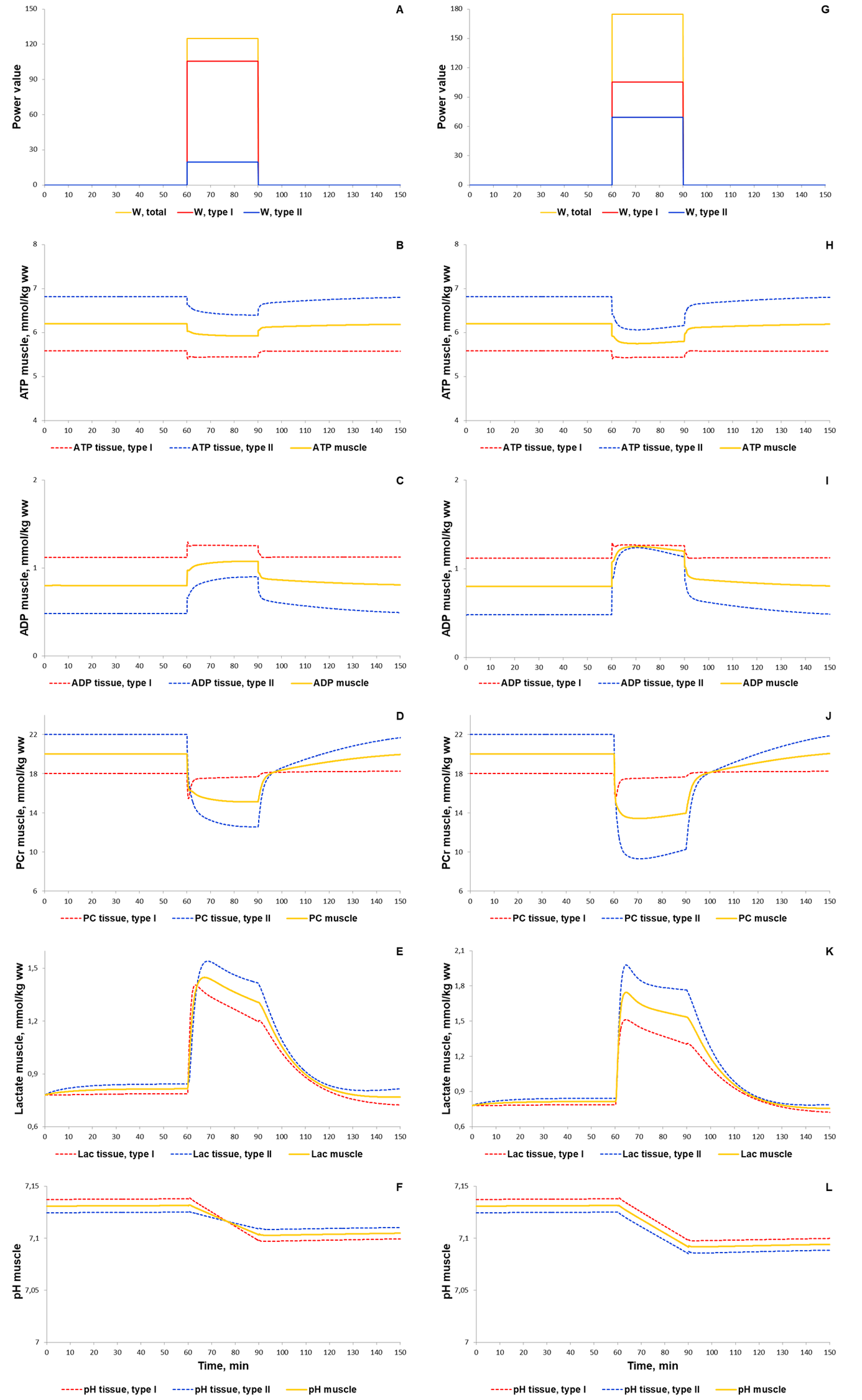
To validate the model we investigated a dynamic behaviour of the system in response to diverse single-bout aerobic exercises and compared them with published experimental data. Initially, we quantitatively estimated biochemical responses of essential metabolic variables (ATP, ADP, PCr, lactate concentrations and pH in muscle fibers type I and II) in the incremental ramp test which is a commonly used approach to evaluate aerobic capacity. In our simulation, muscle fibres type I are recruited after the beginning of exercise, while fibre type II - only at power over 24% of VO2max (6 min after exercise onset, Fig. 1A). Increasing the power during the test effects on various physiological variables like the volume of muscle containing fibre type I and/or II, blood flow as well as of transport and metabolic fluxes in both fibre types (Fig. 1). The model simulations reasonably well correspond to experimental measurements (Roussel et al., 2003; Greiner et al., 2007; Cannon et al., 2013) [1][2] [3] obtained in studies with the exercise mode (Fig. 2). However, it is worth to note that the current version of the model does not take into account an effect of the muscle exhaustion during the incremental ramp test which is physiologically observed and provides a direction for the model improvements.
|
|
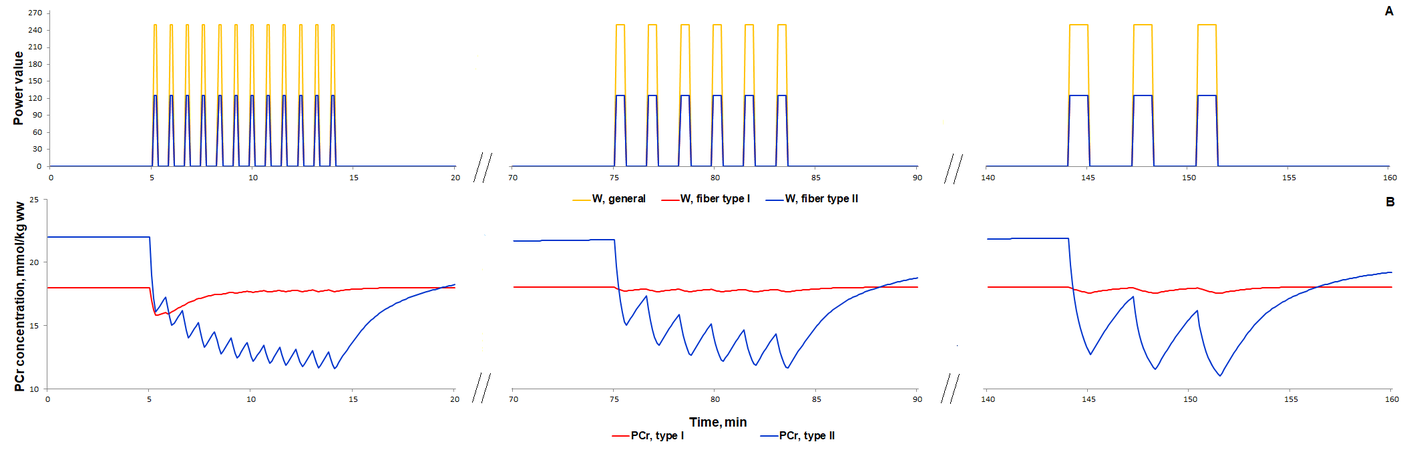
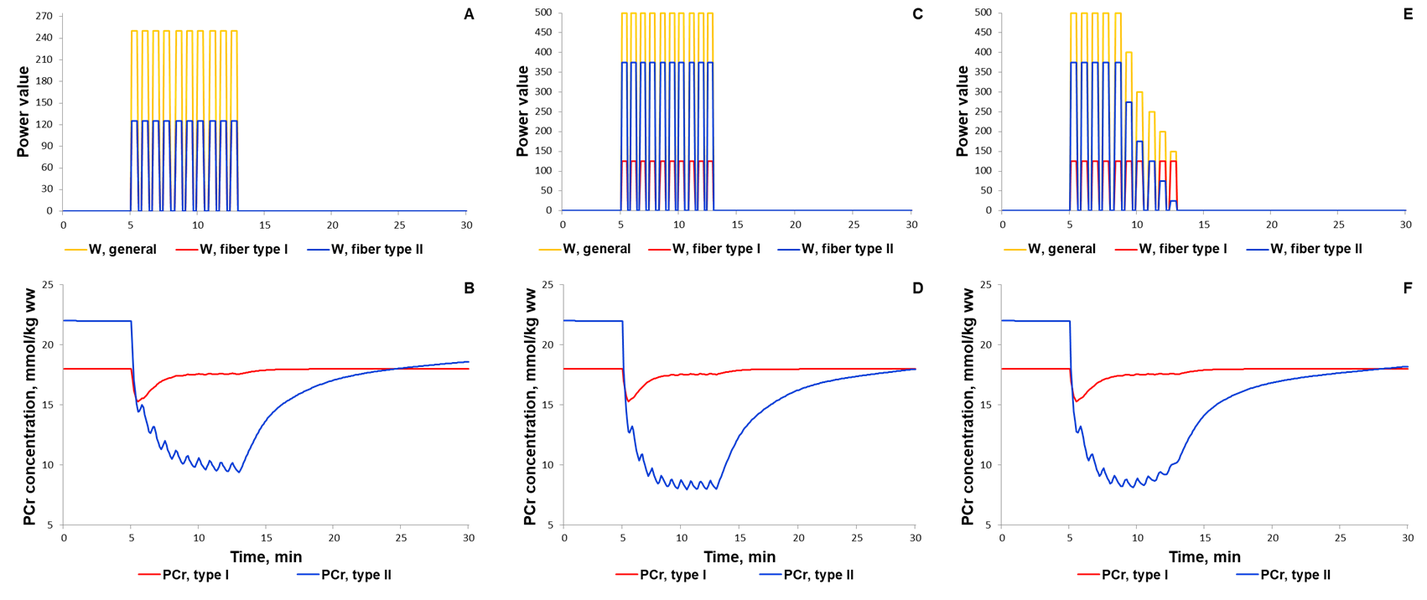
For additional verification of the model, we simulated responses to an alternative exercise mode that is a high-intensity intermittent exercise (Fig. 3, 4). As a result, the model qualitatively reproduces dynamic changes in PCr concentration during high-intensity intermittent exercise with different work:recovery durations.
|
The first scenario to reproduce experimental PCr measurements in Kappenstein with coathours study (Kappenstein et al., 2013)[9] used the external power which is equal to 250, while the parameter value was doubled in the second simulated scenario. The maximal value of the parameter was unchangeable on each bout of 30-s exercise separated by 20-s recovery. Whereas the external power was gradually decreased after the fifth bout (Fig. 4E) due to physical exhaustion of the volunteered subject. It has been predicted that gradual exhaustion or reduction in the external power after a certain exercise bout much better reproduces experimental measurements on phosphocreatine dynamics in skeletal muscle than simulations with constant maximal power value on each bout (Kappenstein et al., 2013)[9].
|
Simulation of dynamic responses at metabolic and gene regulatory levels induced by low- and moderate intensity exercises
At the next step of the model validation, we predicted dynamic changes of the same biochemical variables, an activation of both signaling molecules (AMPK and Ca2+-dependent proteins) and transcription factor (CREB1), as well as expression of genes (NR4A3, NR4A2, PPARGC1A) in response to low (50% VO2max) and moderate intensity (70% VO2max) continuous exercises. During this type of the exercise mode metabolic fluxes varied by means of recruitment of both muscle fibers type I, and type II. Comparison of the model simulations with experimental data (Krustrup et al., 2004; Barker et al., 2008; Cannon et al., 2014; Fiedler et al., 2016; Bartlett et al., 2020)[4][5][6][10][7] has shown that the model reasonably well reproduces dynamic behaviour of the muscle metabolism during the exercise (Fig. 2 and 5).
|
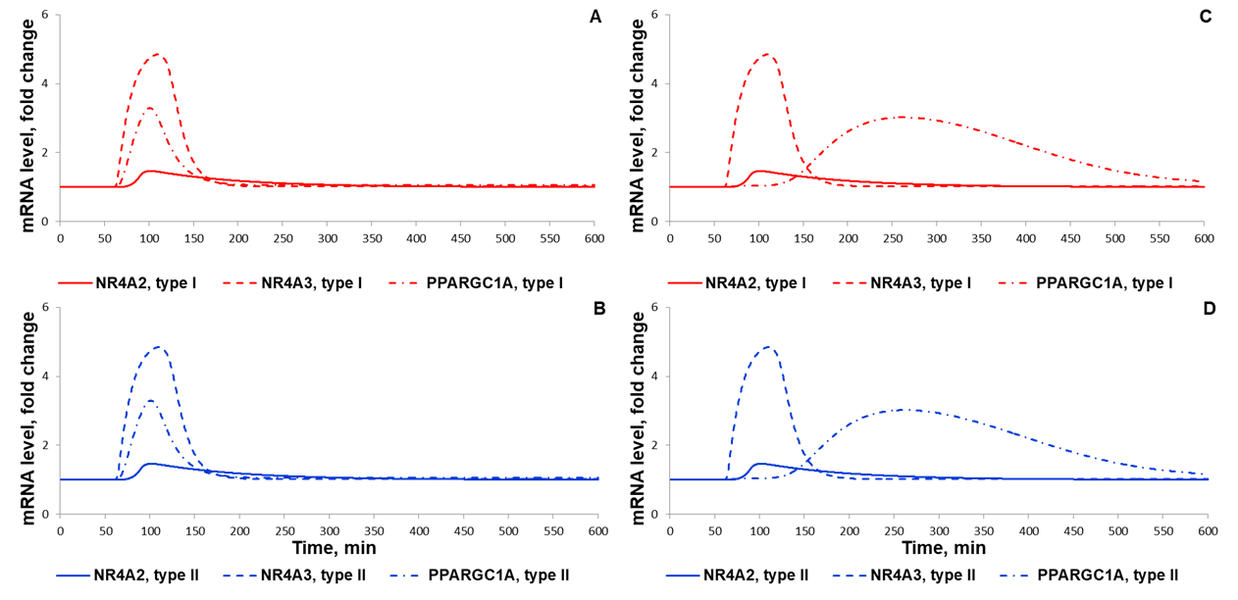
Development of the integrated model incorporating signaling and gene expression modules enables to simulate expression dynamics of early and delayed response genes on mRNA levels (Fig. 6). Numerical analysis of the model enabled to reveal crucial steps in this signal transduction pathway for the adaptation and demonstrated the necessity of consideration of additional transcription factors modulating transcription of genes with delayed response in order to adequately reproduce gene expression data that were taken in human vastus lateralis muscle during and after acute cycling exercise (Fig. 6C-D). Bioinformatics analysis of the original transcriptomics data, in turn, proposed that CREB-like proteins from FOS and JUN families forming heterodimer complexes with transcription factor CREB1 are indeed these intermediate regulators of genes with delayed response (Akberdin et al., 2020)[11].
|
Discussion
The Integrated modular model comprises three hierarchical levels (metabolic, signaling pathways and regulation of gene expression)
We have previously developed a multi-compartmental mathematical model describing the dynamics of intracellular species concentrations and fluxes in human muscle at rest and intracellular metabolic rearrangements in exercising skeletal muscles during an aerobic exercise on a cycle ergometer (Kiselev et al., 2019)[12]. As an initial model for this study, we have used a more complex model of energy metabolism in the human skeletal muscle developed by Li and coauthors and considered two types of the muscle fibers (Li et al., 2012)[13]. We have proposed a modular representation of the complex model using BioUML platform (Kolpakov et al., 2019)[14]. The modular representation provides the possibility of rapid expansion and modification of the model compartments to account for the complex organization of muscle cells and the limitations of the rate of diffusion of metabolites between intracellular compartments. This feature allowed us to integrate modules of signalling pathways modulating downstream regulatory processes of early response and genes with delayed response during the exercise and recovery. The parametric fitting of the modular model to published experimental data (Krustrup et al., 2004; Greiner et al., 2007; Barker et al., 2008; Cannon et al., 2014; Bartlett et al., 2020)[4][2][5][6][7] showed the validity of the modular modeling approach implemented in BioUML. Furthermore, the integrated modular model provides an absolutely novel in silico platform to predict molecular responses of the human skeletal muscle cells to diverse modes of the exercise on levels of metabolic, signaling pathways and gene expression regulation experimental precise measurements of which are currently methodologically limited or even remain elusive.
Model constraints and further ways for the development
GS activity is regulated through multiple mechanisms, including feedbacks mediated by glycogen, blood glucose concentration,rate of glucose uptake, insulin, epinephrine and GS phosphorylation state (Jensen & Lai, 2009; Prats et al., 2009; Jensen et al., 2012b; Palm et al., 2013)[15][16][17][18]. However, in the current model GS activity depends on glycogen content only. In our model, the post-exercise glycogen synthesis is lower than estimated in the majority of studies (Casey et al., 1995; Jentjens & Jeukendrup, 2003; Burke et al., 2017)[19][20][21] because many factors are omitted, such as feeding and associated rises in blood glucose concentration, rate of glucose uptake, sensitivity to and changes in insulin, etc. At the same time, glycogen synthesis is higher than observed during exercise recovery in a fasted state (Maehlum & Hermansen, 1978)[22].
References
- Kolpakov F, Akberdin I, Kashapov T, Kiselev L, Kolmykov S, Kondrakhin Y, Kutumova E, Mandrik N, Pintus S, Ryabova A, Sharipov R, Yevshin I, and Kel A. BioUML: an integrated environment for systems biology and collaborative analysis of biomedical data. Nucleic Acids Res. 2019 Jul 2;47(W1):W225-W233. DOI:10.1093/nar/gkz440 |
- Kolpakov F, Akberdin I, Kashapov T, Kiselev L, Kolmykov S, Kondrakhin Y, Kutumova E, Mandrik N, Pintus S, Ryabova A, Sharipov R, Yevshin I, and Kel A. BioUML: an integrated environment for systems biology and collaborative analysis of biomedical data. Nucleic Acids Res. 2019 Jul 2;47(W1):W225-W233. DOI:10.1093/nar/gkz440 |
- Roussel M, Mattei JP, Le Fur Y, Ghattas B, Cozzone PJ, and Bendahan D. Metabolic determinants of the onset of acidosis in exercising human muscle: a 31P-MRS study. J Appl Physiol (1985). 2003 Mar;94(3):1145-52. DOI:10.1152/japplphysiol.01024.2000 |