Difference between revisions of "Ca-CaM-AMPK signaling models"
| Line 8: | Line 8: | ||
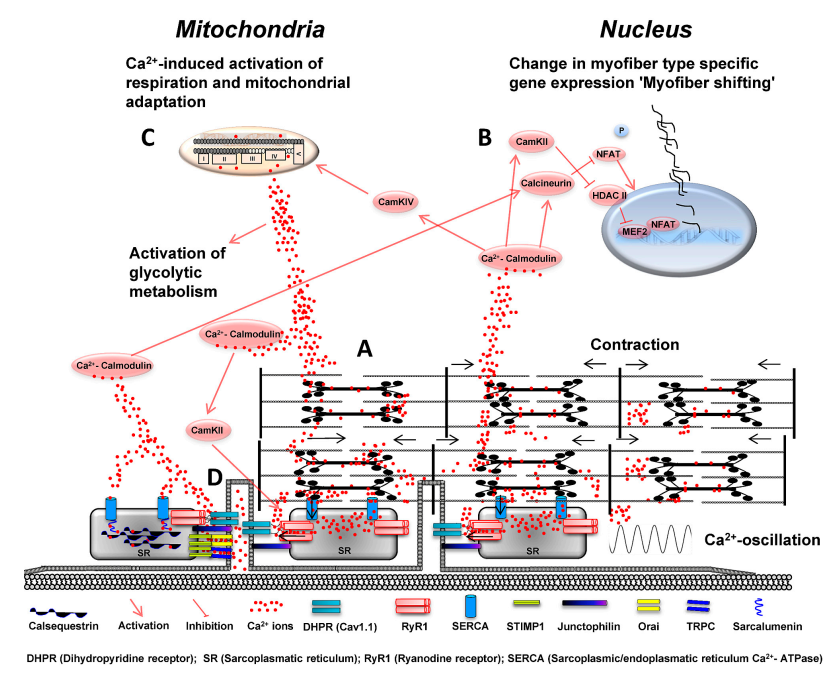
<span style="font-size: 90%"> '''Figure 1 from (Gehlert et al., 2015)''' <cite>1</cite> (a) Voltage-dependent activation of the dihidropyridine receptor (DHPR-Cav1.1) facilitates the release of Ca<sup>2+</sup> ions out of the sarcoplasmatic reticulum (SR), which critically regulates skeletal muscle contraction. Reuptake of Ca<sup>2+</sup> ions in the SR controls skeletal muscle relaxation and is mainly regulated by ATP-dependent sarcoplasmic/endoplasmic reticulum calcium ATPase pumps (SERCA1/2). Increased neuromuscular activity establishes an oscillating pattern of Ca<sup>2+</sup> ion levels and causes elevated sarcoplasmic Ca<sup>2+</sup> ion concentrations in the microenvironment of myofibrils; (b) Increasing levels of Ca2+ ions in the sarcoplasm bind to and activate calmodulin (CaM) which regulates activation of calcineurin and calmodulin kinase II and IV. Calmodulin kinase II (CaMKII) contributes to the phosphorylation of ryanodine receptor 1 (RyR1) which increases RyR1 channel activity and open probability. CaMKII further inhibits histone deacetylase II (HDACII) and increases nuclear abundance of myocyte enhancer factor 2 (MEF2). Calcineurin (CaN) dephosphorylates nuclear factor of activated T-cells (NFAT) hereby regulating its nuclear localization. NFAT and MEF2 facilitate the increased expression of “slow genes” coding protein isoforms of the oxidative fiber type; (c) CaMKIV increases the expression of mitochondrial genes, which contributes to mitochondrial adaptation. Free Ca<sup>2+</sup> ions also directly stimulate or inhibit Ca<sup>2+</sup> release via RyR1 in dependency of their luminal and sarcoplasmic Ca<sup>2+</sup> concentration. Ca<sup>2+</sup> ions further co-regulate the activation of energy metabolism by activating mitochondrial respiration and increasing the activity of glycolytic enzymes in sarcoplasm; and (d) store-operated calcium entry (SOCE) is regulated by stromal interaction molecule 1 (STIM1) which senses declined Ca<sup>2+</sup> ion concentrations in the SR. Interaction of STIMP1 with Orai1 and canonical transient receptor potential channels (TRPC) leads to trans-sarcolemmal Ca<sup>2+</sup> influx to increase intracellular Ca<sup>2+</sup> levels upon declining Ca<sup>2+</sup> content of the SR. Junctophilin maintains junctional triad integrity by overspanning the space between SR and plasma membrane and supports DHPR and RyR1 interaction. Ca<sup>2+</sup> uptake and handling is enhanced by sarcalumenin which interacts with SERCA channels and calsequestrin. </span> | <span style="font-size: 90%"> '''Figure 1 from (Gehlert et al., 2015)''' <cite>1</cite> (a) Voltage-dependent activation of the dihidropyridine receptor (DHPR-Cav1.1) facilitates the release of Ca<sup>2+</sup> ions out of the sarcoplasmatic reticulum (SR), which critically regulates skeletal muscle contraction. Reuptake of Ca<sup>2+</sup> ions in the SR controls skeletal muscle relaxation and is mainly regulated by ATP-dependent sarcoplasmic/endoplasmic reticulum calcium ATPase pumps (SERCA1/2). Increased neuromuscular activity establishes an oscillating pattern of Ca<sup>2+</sup> ion levels and causes elevated sarcoplasmic Ca<sup>2+</sup> ion concentrations in the microenvironment of myofibrils; (b) Increasing levels of Ca2+ ions in the sarcoplasm bind to and activate calmodulin (CaM) which regulates activation of calcineurin and calmodulin kinase II and IV. Calmodulin kinase II (CaMKII) contributes to the phosphorylation of ryanodine receptor 1 (RyR1) which increases RyR1 channel activity and open probability. CaMKII further inhibits histone deacetylase II (HDACII) and increases nuclear abundance of myocyte enhancer factor 2 (MEF2). Calcineurin (CaN) dephosphorylates nuclear factor of activated T-cells (NFAT) hereby regulating its nuclear localization. NFAT and MEF2 facilitate the increased expression of “slow genes” coding protein isoforms of the oxidative fiber type; (c) CaMKIV increases the expression of mitochondrial genes, which contributes to mitochondrial adaptation. Free Ca<sup>2+</sup> ions also directly stimulate or inhibit Ca<sup>2+</sup> release via RyR1 in dependency of their luminal and sarcoplasmic Ca<sup>2+</sup> concentration. Ca<sup>2+</sup> ions further co-regulate the activation of energy metabolism by activating mitochondrial respiration and increasing the activity of glycolytic enzymes in sarcoplasm; and (d) store-operated calcium entry (SOCE) is regulated by stromal interaction molecule 1 (STIM1) which senses declined Ca<sup>2+</sup> ion concentrations in the SR. Interaction of STIMP1 with Orai1 and canonical transient receptor potential channels (TRPC) leads to trans-sarcolemmal Ca<sup>2+</sup> influx to increase intracellular Ca<sup>2+</sup> levels upon declining Ca<sup>2+</sup> content of the SR. Junctophilin maintains junctional triad integrity by overspanning the space between SR and plasma membrane and supports DHPR and RyR1 interaction. Ca<sup>2+</sup> uptake and handling is enhanced by sarcalumenin which interacts with SERCA channels and calsequestrin. </span> | ||
| + | |||
<p align=justify> To ensure sustained contractility of skeletal muscle, the generation of ATP has to match the demands during contraction. A major metabolic pathway in skeletal muscle that provides a high amount of ATP generation per time is the anaerobic glycolysis which converts one molecule glucose to two molecules pyruvate or lactate and two molecules ATP and, in case of glycogen utilization, three molecules ATP per molecule glycogen (Baker et al., 2010) <cite>2</cite>. The regulation of glucose or glycogen breakdown is relatively short-stepped and requires fewer enzymatic driven reactions when compared to aerobic oxidation (e.g., free fatty acids). Ca<sup>2+</sup> ions contribute to the regulation of glycolysis as they affect the enzymatic speed of crucial enzymes of the glycolysis (Schonekess et al., 1995) <cite>3</cite>. Glycogen degradation to pyruvate requires glycogenphosphorylase (GP) which converts one molecule of glycogen to glucose-1-phosphate and primes its further degradation via glycolysis to lactate. The phosphorylation and activation of GPL depends on the activity of the enzyme phosphorylase kinase (PhK). Years ago, it was demonstrated that the important Ca<sup>2+</sup>-binding molecules CaM and troponin C regulate the activity of PhK in interplay with Ca<sup>2+</sup> ions and the phosphorylation by PKA (Cohen, 1980) <cite>4</cite>. PhK in its unphosphorylated form (PhK b) form is relatively inactive when Ca<sup>2+</sup> concentration is low. PKA can phosphorylate PLK on its β-subunit transforming it to its active form (PhK a). However, dependent on Ca<sup>2+</sup> concentration, Ca<sup>2+</sup> ions bind to the δ-subunit of PhK which has a high sequence homology to calmodulin. This mediates an important step in the activation of PhK, however, the additional interaction of PhK with sarcomeric troponin-c seems to be required for the further activation of PhK. The muscle specific isoform of phosphofructokinase (PFK-M) is the most important pacemaker of glycolysis rate. It catalyzes the reaction from fructose 6 phosphate to fructose 1–6 bisphosphate which together with AMP allosterically regulate PFK activity in contracting muscle. Ca<sup>2+</sup> ions are able to modulate PFK activity by the Ca<sup>2+</sup>-dependent activation of CaM which interacts with PFK (Sola-Penna et al., 2010) <cite>5</cite>. PFK monomers have two binding sites for CaM. CaM binding to the high affinity site of PFK forms the generation of stable PFK dimers which exhibit increased catalytic activity of PFK, in part preventing allosteric inhibition of the enzyme, e.g., by ATP, citrate and lactate. The formerly described regulations facilitate the full activation of PhK and contribute to increased PFK activity via increased abundance of Ca<sup>2+</sup>. Hence, these Ca<sup>2+</sup>-dependent mechanisms serve as an important contribution to coordinate the onset of muscle contractions with mechanisms that augment energy metabolism in working muscle.</p> | <p align=justify> To ensure sustained contractility of skeletal muscle, the generation of ATP has to match the demands during contraction. A major metabolic pathway in skeletal muscle that provides a high amount of ATP generation per time is the anaerobic glycolysis which converts one molecule glucose to two molecules pyruvate or lactate and two molecules ATP and, in case of glycogen utilization, three molecules ATP per molecule glycogen (Baker et al., 2010) <cite>2</cite>. The regulation of glucose or glycogen breakdown is relatively short-stepped and requires fewer enzymatic driven reactions when compared to aerobic oxidation (e.g., free fatty acids). Ca<sup>2+</sup> ions contribute to the regulation of glycolysis as they affect the enzymatic speed of crucial enzymes of the glycolysis (Schonekess et al., 1995) <cite>3</cite>. Glycogen degradation to pyruvate requires glycogenphosphorylase (GP) which converts one molecule of glycogen to glucose-1-phosphate and primes its further degradation via glycolysis to lactate. The phosphorylation and activation of GPL depends on the activity of the enzyme phosphorylase kinase (PhK). Years ago, it was demonstrated that the important Ca<sup>2+</sup>-binding molecules CaM and troponin C regulate the activity of PhK in interplay with Ca<sup>2+</sup> ions and the phosphorylation by PKA (Cohen, 1980) <cite>4</cite>. PhK in its unphosphorylated form (PhK b) form is relatively inactive when Ca<sup>2+</sup> concentration is low. PKA can phosphorylate PLK on its β-subunit transforming it to its active form (PhK a). However, dependent on Ca<sup>2+</sup> concentration, Ca<sup>2+</sup> ions bind to the δ-subunit of PhK which has a high sequence homology to calmodulin. This mediates an important step in the activation of PhK, however, the additional interaction of PhK with sarcomeric troponin-c seems to be required for the further activation of PhK. The muscle specific isoform of phosphofructokinase (PFK-M) is the most important pacemaker of glycolysis rate. It catalyzes the reaction from fructose 6 phosphate to fructose 1–6 bisphosphate which together with AMP allosterically regulate PFK activity in contracting muscle. Ca<sup>2+</sup> ions are able to modulate PFK activity by the Ca<sup>2+</sup>-dependent activation of CaM which interacts with PFK (Sola-Penna et al., 2010) <cite>5</cite>. PFK monomers have two binding sites for CaM. CaM binding to the high affinity site of PFK forms the generation of stable PFK dimers which exhibit increased catalytic activity of PFK, in part preventing allosteric inhibition of the enzyme, e.g., by ATP, citrate and lactate. The formerly described regulations facilitate the full activation of PhK and contribute to increased PFK activity via increased abundance of Ca<sup>2+</sup>. Hence, these Ca<sup>2+</sup>-dependent mechanisms serve as an important contribution to coordinate the onset of muscle contractions with mechanisms that augment energy metabolism in working muscle.</p> | ||
| Line 18: | Line 19: | ||
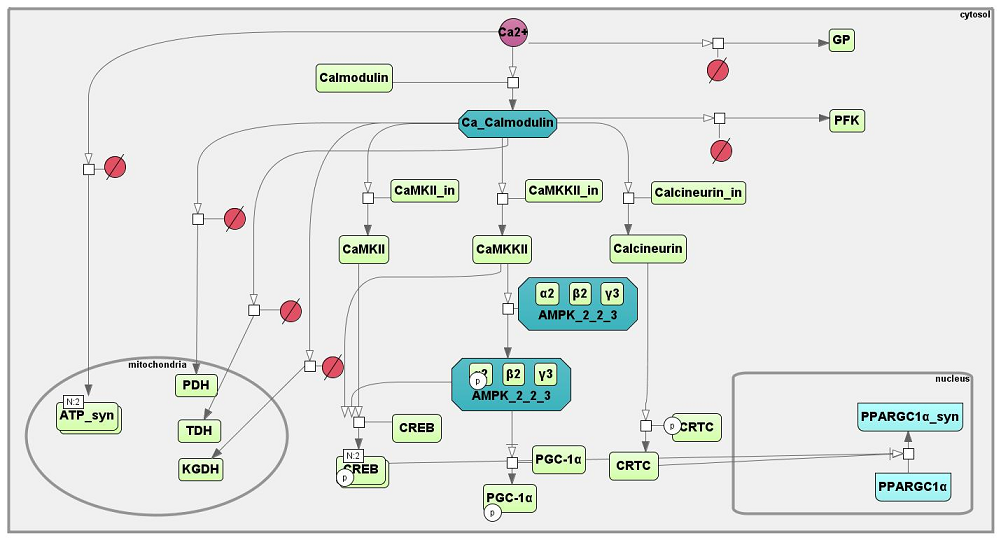
<span style="font-size: 90%"> '''Figure 2''' Schematic diagramme of Ca-CaM-AMPK signaling pathway designed in SBGN standard using BioUML tool. All abbreviations for objects in the main text. </span> | <span style="font-size: 90%"> '''Figure 2''' Schematic diagramme of Ca-CaM-AMPK signaling pathway designed in SBGN standard using BioUML tool. All abbreviations for objects in the main text. </span> | ||
| + | |||
===Published models=== | ===Published models=== | ||
| + | <p align=justify> Calcium acts as a ubiquitous messenger in eukaryotic cells, playing an essential role in a diverse range of processes including cell proliferation, muscle contraction, and apoptosis. Ca<sup>2+</sup> also plays a key role in controlling the | ||
| + | The orchestra of Ca<sup>2+</sup> signaling mechanisms in skeletal muscle determines a multitude of cellular processes. Already the initiation of muscle contraction at the neuromuscular junction is a Ca<sup>2+</sup>-dependent process at the motor endplate inducing a change in membrane polarization and a subsequent opening of L-type Ca<sup>2+</sup> channels triggering the release of Ca<sup>2+</sup> from the sarcoplasmatic reticulum (SR). This mechanism allows a distinct rise of cytosolic Ca<sup>2+</sup> concentration that initiates actin/myosin interaction and movement of the myosin head. To facilitate the interplay of contraction and relaxation the SR is provided by several Ca<sup>2+</sup> transport and binding molecules which are adjusted by a multitude of regulatory molecules. ATP production and hence energy supply of contracting muscle is also regulated by Ca<sup>2+</sup>-dependent enhancement of glycolytic enzyme activity and mitochondrial respiration. The high plasticity of skeletal muscle is enabled by Ca<sup>2+</sup>-dependent regulation of gene expression, translation and posttranslational processes including protein degradation (Gehlert et al., 2015) <cite>1</cite> (Figure 1).</p> | ||
Revision as of 19:21, 22 February 2019
Introduction
Calcium (Ca2+) plays a pivotal role in almost all cellular processes and ensures the functionality of an organism. In skeletal muscle fibers, Ca2+ is critically involved in the innervation of skeletal muscle fibers that results in the exertion of an action potential along the muscle fiber membrane, the prerequisite for skeletal muscle contraction. Furthermore and among others, Ca2+ regulates also intracellular processes, such as myosin-actin cross bridging, protein synthesis, protein degradation and fiber type shifting by the control of Ca2+-sensitive proteases and transcription factors, as well as mitochondrial adaptations, plasticity and respiration. These data highlight the overwhelming significance of Ca2+ ions for the integrity of skeletal muscle tissue. While the fast and acute oscillation of free Ca2+ levels in skeletal muscle is the major step in initiation of muscle contraction and relaxation, slower shifts of cytosolic Ca2+ levels are important contributors in the regulation of skeletal muscle plasticity by activation of specific signaling pathways such as the calmodulin/calcineurin signaling pathway (Gehlert et al., 2015) [1]. Computational modelling is likely to play an important role in analysing the quantitative behaviour of such pathways, in turn providing data for the basis of potential therapeutic drug design. On this page we would like to summarize all developed mathematical models dedicated to this topic of the research.
Ca-CaM-AMPK signaling pathway
The orchestra of Ca2+ signaling mechanisms in skeletal muscle determines a multitude of cellular processes. Already the initiation of muscle contraction at the neuromuscular junction is a Ca2+-dependent process at the motor endplate inducing a change in membrane polarization and a subsequent opening of L-type Ca2+ channels triggering the release of Ca2+ from the sarcoplasmatic reticulum (SR). This mechanism allows a distinct rise of cytosolic Ca2+ concentration that initiates actin/myosin interaction and movement of the myosin head. To facilitate the interplay of contraction and relaxation the SR is provided by several Ca2+ transport and binding molecules which are adjusted by a multitude of regulatory molecules. ATP production and hence energy supply of contracting muscle is also regulated by Ca2+-dependent enhancement of glycolytic enzyme activity and mitochondrial respiration. The high plasticity of skeletal muscle is enabled by Ca2+-dependent regulation of gene expression, translation and posttranslational processes including protein degradation (Gehlert et al., 2015) [1] (Figure 1).
Figure 1 from (Gehlert et al., 2015) [1] (a) Voltage-dependent activation of the dihidropyridine receptor (DHPR-Cav1.1) facilitates the release of Ca2+ ions out of the sarcoplasmatic reticulum (SR), which critically regulates skeletal muscle contraction. Reuptake of Ca2+ ions in the SR controls skeletal muscle relaxation and is mainly regulated by ATP-dependent sarcoplasmic/endoplasmic reticulum calcium ATPase pumps (SERCA1/2). Increased neuromuscular activity establishes an oscillating pattern of Ca2+ ion levels and causes elevated sarcoplasmic Ca2+ ion concentrations in the microenvironment of myofibrils; (b) Increasing levels of Ca2+ ions in the sarcoplasm bind to and activate calmodulin (CaM) which regulates activation of calcineurin and calmodulin kinase II and IV. Calmodulin kinase II (CaMKII) contributes to the phosphorylation of ryanodine receptor 1 (RyR1) which increases RyR1 channel activity and open probability. CaMKII further inhibits histone deacetylase II (HDACII) and increases nuclear abundance of myocyte enhancer factor 2 (MEF2). Calcineurin (CaN) dephosphorylates nuclear factor of activated T-cells (NFAT) hereby regulating its nuclear localization. NFAT and MEF2 facilitate the increased expression of “slow genes” coding protein isoforms of the oxidative fiber type; (c) CaMKIV increases the expression of mitochondrial genes, which contributes to mitochondrial adaptation. Free Ca2+ ions also directly stimulate or inhibit Ca2+ release via RyR1 in dependency of their luminal and sarcoplasmic Ca2+ concentration. Ca2+ ions further co-regulate the activation of energy metabolism by activating mitochondrial respiration and increasing the activity of glycolytic enzymes in sarcoplasm; and (d) store-operated calcium entry (SOCE) is regulated by stromal interaction molecule 1 (STIM1) which senses declined Ca2+ ion concentrations in the SR. Interaction of STIMP1 with Orai1 and canonical transient receptor potential channels (TRPC) leads to trans-sarcolemmal Ca2+ influx to increase intracellular Ca2+ levels upon declining Ca2+ content of the SR. Junctophilin maintains junctional triad integrity by overspanning the space between SR and plasma membrane and supports DHPR and RyR1 interaction. Ca2+ uptake and handling is enhanced by sarcalumenin which interacts with SERCA channels and calsequestrin.
To ensure sustained contractility of skeletal muscle, the generation of ATP has to match the demands during contraction. A major metabolic pathway in skeletal muscle that provides a high amount of ATP generation per time is the anaerobic glycolysis which converts one molecule glucose to two molecules pyruvate or lactate and two molecules ATP and, in case of glycogen utilization, three molecules ATP per molecule glycogen (Baker et al., 2010) [2]. The regulation of glucose or glycogen breakdown is relatively short-stepped and requires fewer enzymatic driven reactions when compared to aerobic oxidation (e.g., free fatty acids). Ca2+ ions contribute to the regulation of glycolysis as they affect the enzymatic speed of crucial enzymes of the glycolysis (Schonekess et al., 1995) [3]. Glycogen degradation to pyruvate requires glycogenphosphorylase (GP) which converts one molecule of glycogen to glucose-1-phosphate and primes its further degradation via glycolysis to lactate. The phosphorylation and activation of GPL depends on the activity of the enzyme phosphorylase kinase (PhK). Years ago, it was demonstrated that the important Ca2+-binding molecules CaM and troponin C regulate the activity of PhK in interplay with Ca2+ ions and the phosphorylation by PKA (Cohen, 1980) [4]. PhK in its unphosphorylated form (PhK b) form is relatively inactive when Ca2+ concentration is low. PKA can phosphorylate PLK on its β-subunit transforming it to its active form (PhK a). However, dependent on Ca2+ concentration, Ca2+ ions bind to the δ-subunit of PhK which has a high sequence homology to calmodulin. This mediates an important step in the activation of PhK, however, the additional interaction of PhK with sarcomeric troponin-c seems to be required for the further activation of PhK. The muscle specific isoform of phosphofructokinase (PFK-M) is the most important pacemaker of glycolysis rate. It catalyzes the reaction from fructose 6 phosphate to fructose 1–6 bisphosphate which together with AMP allosterically regulate PFK activity in contracting muscle. Ca2+ ions are able to modulate PFK activity by the Ca2+-dependent activation of CaM which interacts with PFK (Sola-Penna et al., 2010) [5]. PFK monomers have two binding sites for CaM. CaM binding to the high affinity site of PFK forms the generation of stable PFK dimers which exhibit increased catalytic activity of PFK, in part preventing allosteric inhibition of the enzyme, e.g., by ATP, citrate and lactate. The formerly described regulations facilitate the full activation of PhK and contribute to increased PFK activity via increased abundance of Ca2+. Hence, these Ca2+-dependent mechanisms serve as an important contribution to coordinate the onset of muscle contractions with mechanisms that augment energy metabolism in working muscle.
Ca2+ influx into mitochondria has been shown to result in increased energy conversion potential which is necessary in the maintenance of energetic homeostasis in contracting muscle (Korzeniewski, 2007) [6]. Recent data support this notion of Ca2+-activated muscle oxidative phosphorylation cascade. It could be shown that Ca2+ increased the conductance of complex IV, complexes I + III, ATP production/transport, and fuel transport/dehydrogenases (Glancy et al., 2013) [7]. Ca2+ concentration has also been shown to directly stimulate ATP production through activation of the F1F0-ATP synthase at least in cardiac muscle (Territo et al., 2000) [8]. Extrapolation of these data to the exercising muscle predicts a significant role of Ca2+ concentration in maintaining cellular energy homeostasis. The activation of the electron transport chain in mitochondria by Ca2+ concentration may significantly contribute to the Ca2+ stimulation of ATP production during exercise (Glancy et al., 2013) [7].
Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) is a key regulator of mitochondrial biogenesis, angiogenesis, as well as fat and carbohydrate metabolism in skeletal muscle (Olesen et al., 2010, Popov et al., 2015) [9] [10]. Mouse and human skeletal muscle expresses several PGC-1α (PPARGC1A) gene isoforms originating from the canonical (PGC-1α-a mRNA) and alternative (PGC-1α-b and PGC-1α-c mRNA) promoters (Miura et al., 2008, Yoshioka et al., 2009) [11] [12]. Alternative splicing at the intron between exons 6 and 7 can generate N-truncated (NT) PGC-1α isoforms (Zhang et al., 2009) [13], which possess unique properties, different to those of full-length isoforms (Thom et al., 2014) [14]. Thus, the PGC-1α gene has the potential to produce at least six transcripts (Chang et al., 2012) [15]. Acute endurance exercise leads to increased PGC-1α gene expression in skeletal muscle. AMP-activated protein kinase (AMPK), p38 mitogen-activated protein kinase (p38 MAPK), Ca2+/calmodulin-dependent protein kinase (CaMKII) and Ca2+/calmodulin-dependent protein kinase kinase β (CaMKKII) appear to be important for regulation of activity-induced PGC-1α gene expression from the canonical promoter (Zhang et al., 2014) [16]. Several groups (Norrbom et al., 2011, Ydfors et al., 2013, Popov et al., 2014) [17] [18] [19] have shown that in human skeletal muscle, acute exercise induces PGC-1α gene expression, mainly via the alternative promoter. Based on studies in rodent skeletal muscle, it was proposed that activation of exercise-induced expression via the alternative promoter is regulated by the beta-2 adrenergic receptor-protein kinase A (PKA)-cAMP response element-binding protein (CREB1) signalling pathway (Chinsomboon et al., 2009, Tadaishi et al., 2011) [20] [21]. Human myoblast (Norrbom et al., 2011) [17] and mouse (Wen et al., 2014) [22] studies showed that AMPK plays a role in the regulation of expression via the alternative promoter. However, another myoblast study (Yoshioka et al., 2009) [12] and a study in isolated rat muscle (Tadaishi et al., 2011) [21] did not confirm this finding. Furthermore, it has been demonstrated that constitutive expression PGC-1α gene occurs via the canonical promoter, independent of exercise intensity and exercise-induced increase of AMPKThr172 phosphorylation level. Expression of PGC-1α gene via the alternative promoter is increased of two orders after exercise. This post-exercise expression is highly dependent on the intensity of exercise. There is an apparent association between expression via the alternative promoter and activation of CREB1 (Popov et al., 2015) [23] (Figure 2).
Figure 2 Schematic diagramme of Ca-CaM-AMPK signaling pathway designed in SBGN standard using BioUML tool. All abbreviations for objects in the main text.
Published models
Calcium acts as a ubiquitous messenger in eukaryotic cells, playing an essential role in a diverse range of processes including cell proliferation, muscle contraction, and apoptosis. Ca2+ also plays a key role in controlling the The orchestra of Ca2+ signaling mechanisms in skeletal muscle determines a multitude of cellular processes. Already the initiation of muscle contraction at the neuromuscular junction is a Ca2+-dependent process at the motor endplate inducing a change in membrane polarization and a subsequent opening of L-type Ca2+ channels triggering the release of Ca2+ from the sarcoplasmatic reticulum (SR). This mechanism allows a distinct rise of cytosolic Ca2+ concentration that initiates actin/myosin interaction and movement of the myosin head. To facilitate the interplay of contraction and relaxation the SR is provided by several Ca2+ transport and binding molecules which are adjusted by a multitude of regulatory molecules. ATP production and hence energy supply of contracting muscle is also regulated by Ca2+-dependent enhancement of glycolytic enzyme activity and mitochondrial respiration. The high plasticity of skeletal muscle is enabled by Ca2+-dependent regulation of gene expression, translation and posttranslational processes including protein degradation (Gehlert et al., 2015) [1] (Figure 1).
References
- Gehlert S, Bloch W, and Suhr F. Ca2+-dependent regulations and signaling in skeletal muscle: from electro-mechanical coupling to adaptation. Int J Mol Sci. 2015 Jan 5;16(1):1066-95. DOI:10.3390/ijms16011066 |
- Baker JS, McCormick MC, and Robergs RA. Interaction among Skeletal Muscle Metabolic Energy Systems during Intense Exercise. J Nutr Metab. 2010;2010:905612. DOI:10.1155/2010/905612 |
- Schönekess BO, Brindley PG, and Lopaschuk GD. Calcium regulation of glycolysis, glucose oxidation, and fatty acid oxidation in the aerobic and ischemic heart. Can J Physiol Pharmacol. 1995 Nov;73(11):1632-40. DOI:10.1139/y95-725 |
- Cohen P. The role of calcium ions, calmodulin and troponin in the regulation of phosphorylase kinase from rabbit skeletal muscle. Eur J Biochem. 1980 Oct;111(2):563-74. DOI:10.1111/j.1432-1033.1980.tb04972.x |
- Sola-Penna M, Da Silva D, Coelho WS, Marinho-Carvalho MM, and Zancan P. Regulation of mammalian muscle type 6-phosphofructo-1-kinase and its implication for the control of the metabolism. IUBMB Life. 2010 Nov;62(11):791-6. DOI:10.1002/iub.393 |
- Korzeniewski B. Regulation of oxidative phosphorylation through parallel activation. Biophys Chem. 2007 Sep;129(2-3):93-110. DOI:10.1016/j.bpc.2007.05.013 |
- Glancy B, Willis WT, Chess DJ, and Balaban RS. Effect of calcium on the oxidative phosphorylation cascade in skeletal muscle mitochondria. Biochemistry. 2013 Apr 23;52(16):2793-809. DOI:10.1021/bi3015983 |
- Territo PR, Mootha VK, French SA, and Balaban RS. Ca(2+) activation of heart mitochondrial oxidative phosphorylation: role of the F(0)/F(1)-ATPase. Am J Physiol Cell Physiol. 2000 Feb;278(2):C423-35. DOI:10.1152/ajpcell.2000.278.2.C423 |
- Olesen J, Kiilerich K, and Pilegaard H. PGC-1alpha-mediated adaptations in skeletal muscle. Pflugers Arch. 2010 Jun;460(1):153-62. DOI:10.1007/s00424-010-0834-0 |
- Popov DV, Lysenko EA, Kuzmin IV, Vinogradova V, and Grigoriev AI. Regulation of PGC-1α Isoform Expression in Skeletal Muscles. Acta Naturae. 2015 Jan-Mar;7(1):48-59.
- Miura S, Kai Y, Kamei Y, and Ezaki O. Isoform-specific increases in murine skeletal muscle peroxisome proliferator-activated receptor-gamma coactivator-1alpha (PGC-1alpha) mRNA in response to beta2-adrenergic receptor activation and exercise. Endocrinology. 2008 Sep;149(9):4527-33. DOI:10.1210/en.2008-0466 |
- Yoshioka T, Inagaki K, Noguchi T, Sakai M, Ogawa W, Hosooka T, Iguchi H, Watanabe E, Matsuki Y, Hiramatsu R, and Kasuga M. Identification and characterization of an alternative promoter of the human PGC-1alpha gene. Biochem Biophys Res Commun. 2009 Apr 17;381(4):537-43. DOI:10.1016/j.bbrc.2009.02.077 |
- Zhang Y, Huypens P, Adamson AW, Chang JS, Henagan TM, Boudreau A, Lenard NR, Burk D, Klein J, Perwitz N, Shin J, Fasshauer M, Kralli A, and Gettys TW. Alternative mRNA splicing produces a novel biologically active short isoform of PGC-1alpha. J Biol Chem. 2009 Nov 20;284(47):32813-26. DOI:10.1074/jbc.M109.037556 |
- Thom R, Rowe GC, Jang C, Safdar A, White JP, and Arany Z. Hypoxic induction of vascular endothelial growth factor (VEGF) and angiogenesis in muscle by N terminus peroxisome proliferator-associated receptor gamma coactivator (NT-PGC)-1α. J Biol Chem. 2015 Aug 7;290(32):19543. DOI:10.1074/jbc.A113.512061 |
- Chang JS, Fernand V, Zhang Y, Shin J, Jun HJ, Joshi Y, and Gettys TW. NT-PGC-1α protein is sufficient to link β3-adrenergic receptor activation to transcriptional and physiological components of adaptive thermogenesis. J Biol Chem. 2012 Mar 16;287(12):9100-11. DOI:10.1074/jbc.M111.320200 |
- Zhang Y, Uguccioni G, Ljubicic V, Irrcher I, Iqbal S, Singh K, Ding S, and Hood DA. Multiple signaling pathways regulate contractile activity-mediated PGC-1α gene expression and activity in skeletal muscle cells. Physiol Rep. 2014 May 1;2(5). DOI:10.14814/phy2.12008 |
- Norrbom J, Sällstedt EK, Fischer H, Sundberg CJ, Rundqvist H, and Gustafsson T. Alternative splice variant PGC-1α-b is strongly induced by exercise in human skeletal muscle. Am J Physiol Endocrinol Metab. 2011 Dec;301(6):E1092-8. DOI:10.1152/ajpendo.00119.2011 |
- Ydfors M, Fischer H, Mascher H, Blomstrand E, Norrbom J, and Gustafsson T. The truncated splice variants, NT-PGC-1α and PGC-1α4, increase with both endurance and resistance exercise in human skeletal muscle. Physiol Rep. 2013 Nov;1(6):e00140. DOI:10.1002/phy2.140 |
- Popov DV, Bachinin AV, Lysenko EA, Miller TF, and Vinogradova OL. Exercise-induced expression of peroxisome proliferator-activated receptor γ coactivator-1α isoforms in skeletal muscle of endurance-trained males. J Physiol Sci. 2014 Sep;64(5):317-23. DOI:10.1007/s12576-014-0321-z |
- Chinsomboon J, Ruas J, Gupta RK, Thom R, Shoag J, Rowe GC, Sawada N, Raghuram S, and Arany Z. The transcriptional coactivator PGC-1alpha mediates exercise-induced angiogenesis in skeletal muscle. Proc Natl Acad Sci U S A. 2009 Dec 15;106(50):21401-6. DOI:10.1073/pnas.0909131106 |
- Tadaishi M, Miura S, Kai Y, Kawasaki E, Koshinaka K, Kawanaka K, Nagata J, Oishi Y, and Ezaki O. Effect of exercise intensity and AICAR on isoform-specific expressions of murine skeletal muscle PGC-1α mRNA: a role of β₂-adrenergic receptor activation. Am J Physiol Endocrinol Metab. 2011 Feb;300(2):E341-9. DOI:10.1152/ajpendo.00400.2010 |
- Wen X, Wu J, Chang JS, Zhang P, Wang J, Zhang Y, Gettys TW, and Zhang Y. Effect of exercise intensity on isoform-specific expressions of NT-PGC-1 α mRNA in mouse skeletal muscle. Biomed Res Int. 2014;2014:402175. DOI:10.1155/2014/402175 |
- Popov DV, Lysenko EA, Vepkhvadze TF, Kurochkina NS, Maknovskii PA, and Vinogradova OL. Promoter-specific regulation of PPARGC1A gene expression in human skeletal muscle. J Mol Endocrinol. 2015 Oct;55(2):159-68. DOI:10.1530/JME-15-0150 |